Transforming growth factor-β1 (TGF-β1) has potent physiologic and pathologic effects on a variety of cell types at subnanomolar concentrations. Platelets contain 40 times as much TGF-β1 as other cells and secrete it as an inactive (latent) form in complex with latency-associated peptide (LAP), which is disulfide bonded via Cys33 to latent TGF-β binding protein 1 (LTBP-1). Little is known about how latent TGF-β1 becomes activated in vivo. Here we show that TGF-β1 released from platelets or fibroblasts undergoes dramatic activation when subjected to stirring or shear forces, providing a potential mechanism for physiologic control. Thiol-disulfide exchange appears to contribute to the process based on the effects of thiol-reactive reagents and differences in thiol labeling of TGF-β1 before and after stirring or shear. Activation required the presence of LTBP, as TGF-β1 contained in complex with only LAP could not be activated by stirring when studied as either a recombinant purified protein complex or in the platelet releasates or sera of mice engineered to contain an LAP C33S mutation. Release and activation of latent TGF-β1 in vivo was demonstrated in a mouse model 5 minutes after thrombus formation. These data potentially provide a novel mechanism for in vivo activation of TGF-β1.
Introduction
Transforming growth factor-β1 (TGF-β1) has potent physiologic and pathologic effects on a variety of cell types at subnanomolar concentrations, including cells of the immune and hematopoietic systems, as well as malignant cells and fibroblasts,1 with the latter responding with increased collagen production leading to tissue fibrosis.2 Nearly all cellular TGF-β1 exists, however, in a biologically inactive (latent) form in a noncovalent complex with the remaining portion of its precursor molecule, latency-associated peptide (LAP), which is disulfide bonded to a latent TGF-β binding protein (LTBP-1, 3, or 4), forming the large latent complex (LLC).3 Although much is known about the signaling mechanisms and downstream effects of TGF-β1,4 and several latent TGF-β1 activating mechanisms have been identified in vitro (reviewed in Annes et al3 ), little is known about the physiologic mechanisms that control latent TGF-β1 activation in vivo. Recent data support activation of LLC TGF-β1 via a traction mechanism,3,5,,,,,–11 with LAP binding to integrins αVβ5, αVβ6, and αVβ8, and LTBP-1 binding to extracellular matrix.
Blood platelets are a rich source of TGF-β1, containing 40 to 100 times as much as other cells,12 and releasing it when activated by a variety of agents, including thrombin, which is produced during blood clotting.13,,,,,–19 Virtually all of the TGF-β1 released from platelets is in the LLC.3,16 Because platelet latent TGF-β1 is released into the circulation, and because traction on the molecule has been proposed as a mechanism of latent TGF-β1 activation,5,6 we hypothesized that intravascular shear force may serve as an analog of traction mediated by cellular contraction and contribute to the activation of latent TGF-β1 released from platelets. We therefore tested this hypothesis by both in vitro and in vivo experiments.
Methods
Antibodies and reagents
Anti–TGF-β1 (polyclonal chicken IgY), anti-LAP (polyclonal goat IgG), and anti–LTBP-1 (monoclonal antibody [mAb]; clone 35409) were from R&D Systems (Minneapolis, MN), and a rabbit mAb to phospho-Smad2 and a rabbit polyclonal antibody to Smad2/3 were from Cell Signaling (Boston, MA). Purified human thrombin was from Enzyme Research Lab (South Bend, IN); 3-(N-maleimidylpropionyl)biocytin (MPB) was from Invitrogen (Carlsbad, CA); and glutathione (GSH), iodoacetamide, N-acetylcysteine (NAC), N-ethylmaleimide (NEM), and ferric chloride (FeCl3) were from Sigma-Aldrich (St Louis, MO).
TGF-β1 assays
Both immunologic and functional assays were used to measure TGF-β1. The immunologic enzyme-linked immunosorbent assay (ELISA) used an immobilized mAb specific for the active form of TGF-β1 to capture active TGF-β1 and a chicken polyclonal antibody to detect the captured TGF-β1 (R&D Systems). Active TGF-β1 was measured directly in untreated samples, whereas total TGF-β1 (active + latent) was measured after pretreating the samples with 0.2 volume of 1 N HCl for 20 minutes at room temperature to convert the latent TGF-β1 to active TGF-β1. One functional assay used a mink lung epithelial cell line (MLEC) stably expressing the plasminogen activator inhibitor-1 (PAI-1) promoter attached to a luciferase gene; this assay is based on the observation that PAI-1 gene expression is increased in response to as little as 10 pg/mL TGF-β1.20 Briefly, MLECs (2.5 × 104) were plated in a 96-well tissue culture plate and allowed to adhere for 3 hours. The medium was replaced with 90 μL serum-free medium (DMEM containing antibiotics), and the sample to be tested (10 μL) was added for 18 to 20 hours at 37°C. Luciferase activity driven by the PAI-1 promoter was assayed from cell lysates in an automated luminometer using a luciferase assay system (Promega, Madison, WI). The MLEC assay was used to confirm the results of the ELISA, both of which gave consistent results for the relative relationships between samples, although the absolute values measured from standard curves differed somewhat. In some experiments, latent TGF-β1 activation was also assayed by measuring Smad2 phosphorylation using a mAb specific for phosphorylated Smad2. Hirudin (100 nM) was added to all cell-based assays to prevent thrombin-induced signaling events. In some experiments, samples were incubated with a TGF-β1 neutralizing antibody (chicken IgY; 2 μg/mL) to assess the specificity of TGF-β1 signaling detected by the PAI-1 luciferase and Smad2 phosphorylation assays.
Preparation of human platelet releasate and serum
Platelet isolation and aggregation.
Human studies were approved by the Rockefeller University Institutional Review Board and informed consent was obtained in accordance with the Declaration of Helsinki. Blood was drawn from healthy volunteers after informed consent was obtained using a 19-gauge needle and a syringe containing 0.1 volume of 3.8% citrate. Platelet-rich plasma (PRP) was prepared by centrifuging the blood for 4 minutes at 650g at room temperature (RT). In some cases, freshly isolated units of platelets were obtained from the New York Blood Center (New York, NY). The PRP or the units of platelets were centrifuged at 1200g for 8 minutes at RT. The platelet pellet was washed once in HEPES-buffered modified Tyrode buffer (HBMT; pH 7.4), supplemented with 1 μM PGE1, and recentrifuged at 1200g for 8 minutes at RT. Washed platelets (1 × 109/mL) were resuspended in HBMT, pH 7.4, containing 0.35% BSA, 0.1% dextrose, 1 mM Ca2+, and 1 mM Mg2+, prewarmed to 37°C for 10 minutes, and stimulated with thrombin (0.125 U/mL) for 5 minutes at 37°C. Platelet-free platelet releasates were prepared by centrifugation at 14 000g for 20 minutes at 4°C and assayed for active and total TGF-β1 by ELISA and PAI-1 luciferase assay.
Preparation of serum.
Blood was drawn and transferred immediately into a glass tube without an anticoagulant, followed by incubation at 37°C for 4 hours. The clot was gently removed using a wooden stick, and the serum was collected by centrifuging at 14 000g for 20 minutes at 4°C.
Preparation of conditioned medium from human fibroblasts
Human normal skin fibroblasts (Detroit 551) were obtained from ATCC (Manassas, VA) and grown in DMEM containing 10% FBS with antibiotics for 2 days. Nearly confluent monolayers were washed 3 times with PBS and incubated for 10 minutes at 37°C to remove the remaining serum. The medium was replaced with 1 mL serum-free medium (DMEM containing antibiotics) and incubated for either 10 minutes or 18 hours at 37°C. Conditioned medium was collected by centrifugation at 12 000g for 10 minutes at 4°C.
Activation of latent TGF-β1 by stirring or shear
Effect of stirring.
Platelet releasates or serum or conditioned medium samples (200 μL) were added to 7-mm diameter glass cuvettes containing metal stir bars (5 mm) and stirred at 1200 rpm in an aggregometer (Chrono-Log, Havertown, PA) at 37°C for the indicated time periods and then assayed for active and total TGF-β1.
Effect of shear.
Sera or platelet releasates (200 μL) were placed in a polystyrene plastic plate (Nalge-Nunc International, Roskilde, Denmark) and subjected to a shear rate of 1800 s−1 in a cone and plate device as described by Varon et al.21 Samples were collected at the indicated time points and assayed for active and total TGF-β1.
Labeling free thiols
Proteins containing free cysteine thiols were labeled with MPB as described previously.22 Briefly, MPB (100 μM) was added to platelet supernatants 5 minutes after thrombin stimulation and the samples were stirred for an additional 5 or 120 minutes at 37°C. Residual unreacted MPB was quenched by adding GSH (200 μM). The specificity of MPB labeling for free thiol groups was assessed by pretreating select samples with NEM (1 mM) before adding MPB. In some studies, MPB-labeled proteins were immunoprecipitated (IP) using the indicated antibodies directly coupled to magnetic beads (Dynabeads; Invitrogen). Samples were mixed with SDS sample buffer, heated to 100°C for 3 minutes, and electrophoresed in 8% to 16% gradient Tris-glycine gels (Invitrogen). After transfer of proteins to polyvinylidene fluoride (PVDF) membranes, biotinylated proteins were detected with streptavidin–horseradish peroxidase (SA-HRP) using chemiluminescence (enhanced chemiluminescence [ECL] detection system; Amersham, Pittsburgh, PA). Immunoblots were performed on platelet lysates and releasates using the following antibodies: goat anti-LAP; chicken anti–TGF-β1, and mouse anti–LTBP-1. Proteins were detected by appropriate secondary antibodies conjugated to horseradish peroxidase (HRP; Jackson Laboratories, West Grove, PA) followed by chemiluminescence detection. Band intensities were quantitated using image analysis software (National Institutes of Health [NIH]–Scion Image; Scion, Frederick, MD).
Depletion of free thiol-containing proteins by thiol affinity chromatography
Proteins containing accessible free thiols were depleted from platelet releasates by chromatography on an activated thiopropyl-Sepharose 6B column (GE Healthcare, Piscataway, NJ) as described by Choi et al.23 Briefly, 1 mL thiopropyl-Sepharose 6B or control Sepharose 6B beads were packed in a 1.5 × 7-cm column (PD-10; BioRad, Hercules, CA) and washed with 0.2 M NaCl, 0.1 M Tris/HCl, pH 7.5. Platelet releasate (1 mL) was advanced into the column and incubated for 30 minutes at RT. The column was eluted with HBMT buffer, pH 7.4, and the flow-through was collected.
Smad2 phosphorylation
MLECs were stimulated for 2 hours in serum-free medium with stirred or unstirred platelet releasates in the presence or absence of anti–TGF-β1 neutralizing antibody (anti–TGF-β1; 2 μg/mL). Smad2 phosphorylation was detected by immunoblotting with a mAb specific for phosphorylated Smad2 (P-Smad2), and total Smad2 was detected with a polyclonal antibody that reacts with both phosphorylated and unphosphorylated Smad2.
Mouse experiments
Mice.
Wild-type (C57Bl/6) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice with an LAP C33S mutation that prevents covalent coupling of LAP to LTBP-1 were generated by altering the Tgfb1 codon for C33 to one for S33 by homologous recombination (K.Y., H. Obata, V. Jurukovski, R. Mazzieri, Y. Chen, L. Zilberberg, D. Huso, J. Melamed, P. Prijatelj, V. Todorovic B. Dabovic, and D.B.R., manuscript in preparation). These mutant mice have many features similar to those of Tgfb1–null mice, indicating a severe functional deficiency of TGF-β1, but their abnormalities are not quite as severe as those of Tgfb1–null mice.20,24,25 Mice were housed in a controlled environment (23 ± 2°C; 12-hour light/dark cycles), and fed a standard diet (5001; Purina Mills, Richmond, IN). All experimental procedures conformed to the recommendations of the Guide for the Care and Use of Laboratory Animals26 and approved by either the New York University or the Rockefeller University Animal Care and Use Committee.
Latent TGF-β1 activation in vitro in mouse platelets and serum.
Mouse platelets were isolated from 500 μL blood drawn from the inferior vena cava in a syringe containing 100 μL acid citrate dextrose (ACD), 400 μL mouse HEPES-buffered saline (MHBS) (10 mM HEPES, 165 mM NaCl), and 2 μL 1 mM PGE1. Washed platelets were prepared as described by Lengweiler et al27 and resuspended in HBMT buffer, pH 7.4, containing 0.35% BSA, 0.1% dextrose, 1 mM Ca2+, and 1 mM Mg2+. Platelets were then stimulated with thrombin (0.125 U/mL) for 5 minutes at 37°C, after which cell-free platelet releasates were prepared by centrifugation at 14 000g for 20 minutes at 4°C. Mouse serum was prepared from blood drawn percutaneously from the left ventricle of anesthetized mice under ultrasound guidance (Vevo 770; VisualSonics, Toronto, ON) using a 27-gauge needle and a 1-mL syringe. Blood was transferred to a glass tube and incubated for 4 hours at 37°C, after which the serum was collected by centrifuging at 14 000g for 20 minutes at 4°C. Both platelet releasates and sera (200 μL) were subjected to stirring in a glass cuvette (7 × 50 mm) containing a metal stir bar (1 × 5 mm) for 2 hours at 37°C. Activation of latent TGF-β1 was assayed by the ELISA and PAI-1 luciferase reporter assays.
Latent TGF-β1 activation in vivo in the carotid artery thrombosis model.
Mice were anesthetized with isoflurane and maintained under inhalation anesthesia as previously described.28 Mouse body temperature was monitored with a rectal probe and thermistor (THM100; Indus Industries, Houston, TX) and maintained at 37°C (± 2°C) with a temperature-controlled heating pad (THM100). The left common carotid artery was dissected and a Doppler flow probe (Transonics 0.5; Transonic Systems, Ithaca, NY) was placed on the carotid artery proximal to the bifurcation as previously described.29 Thrombosis was induced by placing a 3-mm piece of filter paper (Whatman no. 1; Whatman, Maidstone, United Kingdom) saturated with 8% FeCl3 on the exposed segment of the carotid artery for 3 minutes. Occlusive thrombi formed uniformly within 3 to 5 minutes and after an additional 5 or 120 minutes, 4-mm arterial segments containing the thrombi were excised. The segments were then opened, and the thrombi were removed and dispersed in 200 μL HBMT buffer, pH 7.4, on ice for 1 hour. After centrifugation at 14 000g for 20 minutes at 4°C, TGF-β1 was measured in the supernatants using the ELISA and/or PAI-1 luciferase reporter assay, without or with neutralization with the anti–TGF-β1 antibody.
Statistical analysis
Data are expressed as means plus or minus SD, with the significance of differences calculated by Student t test.
Results
Stirring dramatically enhances TGF-β1 activation
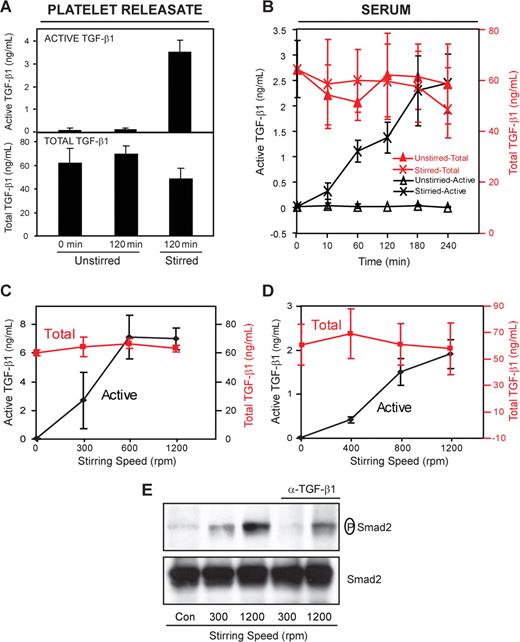
We initially assessed the effect of stirring in an aggregometer cuvette on latent TGF-β1 activation of both platelet releasates and serum. Platelet releasates were produced by stimulating washed human platelets with thrombin (0.125 U/mL) for 5 minutes and separating the supernatant releasate from the platelets by centrifugation. Serum was produced by allowing blood to clot for 4 hours at 37°C and removing the clot by centrifugation. Active TGF-β1 was measured directly in untreated samples, whereas the total TGF-β1 (latent + active) was measured after activating all of the latent TGF-β1 with acid.30 Maximal release of total TGF-β1, accompanied by LAP and LTBP-1, the other 2 components of LLC, occurred within 5 minutes of thrombin stimulation (Figure 1), but only 0.2% of the TGF-β1 was active (Figure 2A). Even after 2 hours at 37°C without stirring, there was virtually no further activation of latent TGF-β1. In sharp contrast, after 2 hours of stirring at 1200 rpm at 37°C, TGF-β1 activity increased more than 30-fold to approximately 7% of the total (active: 3.5 ± 0.5 ng/mL; total: 48 ± 9 ng/mL; n = 3; Figure 2A). This was accompanied by a modest and variable decrease in total TGF-β1 (Figure 2A), which was shown to be due, at least in part, to adsorption of TGF-β1 to the walls of the cuvette (data not shown). Similar results were obtained when platelets were not removed from the releasates before stirring, although the activation reached only approximately 50% of the level achieved with platelet-free releasates after 2 hours of stirring (data not shown). Data obtained using sera were similar, with only 0.02% of the total TGF-β1 (65 ± 11 ng/mL; n = 4) active before stirring and approximately 4% active after 3 hours of stirring (Figure 2B). As with the platelet releasates, even after 3 hours, only 0.03% of the TGF-β1 was active in unstirred samples (Figure 2B). Latent TGF-β1 activation was dependent on the stirring speed, with the platelet releasates and sera demonstrating a plateau of activation between 800 to 1200 rpm (Figure 2C,D). To assess the biologic activity of the TGF-β1 quantified in the ELISA, we measured the ability of the sample to induce Smad2 phosphorylation, one of the known effects of TGF-β1 signaling.4 Phosphorylation of Smad2 correlated with the results of the active TGF-β1 ELISA, showing marked enhancement when samples were stirred (Figure 2E). A neutralizing antibody to TGF-β1 reduced Smad2 phosphorylation by more than 99% at 300 rpm and approximately 70% at 1200 rpm, demonstrating the TGF-β1 specificity of the effect on Smad2 phosphorylation.
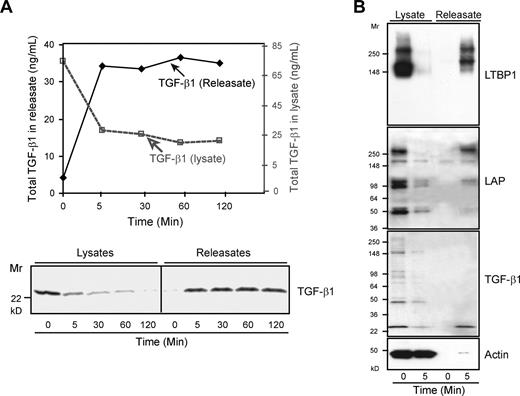
Total TGF-β1 is released as LLCs within 5 minutes after platelet stimulation. Platelets (109 per mL) were stimulated with thrombin (0.125 U/mL) at 37°C for the indicated time periods and centrifuged at 14 000g for 20 minutes at 4°C. Pellets were lysed in buffer containing Triton-X-100 (1%). (A) Total TGF-β1 in platelet lysates and releasates before and after thrombin stimulation for the indicated times was measured by ELISA after acidification (top panel) and immunoblotting with anti–TGF-β1 (bottom panel); results shown are from 1 of more than 10 similar experiments. (B) Both lysates and releasates were immunoblotted with antibodies to LTBP1, LAP, TGF-β1, and actin. The immunoblots demonstrate release of TGF-β1, LAP, and LTBP-1, the 3 components of LLCs. LAP and the upper band of LTBP-1 migrated at Mr approximately 270 kD, consistent with their forming a 1:1 complex. The lower LTBP-1 band of Mr approximately 160-kD identified in the immunoblot has the same Mr reported for free LTBP-1.31 The lower bands identified in the LAP immunoblot have Mr's consistent with precursor molecules that are not covalently coupled to LTBP-1.
Total TGF-β1 is released as LLCs within 5 minutes after platelet stimulation. Platelets (109 per mL) were stimulated with thrombin (0.125 U/mL) at 37°C for the indicated time periods and centrifuged at 14 000g for 20 minutes at 4°C. Pellets were lysed in buffer containing Triton-X-100 (1%). (A) Total TGF-β1 in platelet lysates and releasates before and after thrombin stimulation for the indicated times was measured by ELISA after acidification (top panel) and immunoblotting with anti–TGF-β1 (bottom panel); results shown are from 1 of more than 10 similar experiments. (B) Both lysates and releasates were immunoblotted with antibodies to LTBP1, LAP, TGF-β1, and actin. The immunoblots demonstrate release of TGF-β1, LAP, and LTBP-1, the 3 components of LLCs. LAP and the upper band of LTBP-1 migrated at Mr approximately 270 kD, consistent with their forming a 1:1 complex. The lower LTBP-1 band of Mr approximately 160-kD identified in the immunoblot has the same Mr reported for free LTBP-1.31 The lower bands identified in the LAP immunoblot have Mr's consistent with precursor molecules that are not covalently coupled to LTBP-1.
Stirring enhances latent TGF-β1 activation. (A) Platelet releasate or (B) serum (200 μL) was placed in a glass aggregometer cuvette containing a metal stir bar and incubated at 37°C with and without stirring (1200 rpm) for the indicated time periods. Active TGF-β1 was measured directly in the ELISA, whereas total TGF-β1 (latent + active) was measured after activating latent TGF-β1 by treatment with acid. Stirring dramatically increased the amount of active TGF-β1 in both platelet releasates (3.5 ± 0.5 ng/mL, P < .001; n = 3) and sera (2.5 ± 0.6 ng/mL, P < .001; n = 4). Increasing the stirring speed enhanced latent TGF-β1 activation in both platelet releasates (C) and sera (D) when measured after 2 hours. Error bars represent SD. (E) MLECs were stimulated for 2 hours in serum-free medium with stirred or unstirred platelet releasates in the presence or absence of anti–TGF-β1 neutralizing antibody (α–TGF-β1; 2 μg/mL). Smad2 phosphorylation was detected by immunoblotting with a mAb specific for phosphorylated Smad2 (P-Smad2), and total Smad2 was detected with a polyclonal antibody that reacts with both phosphorylated and unphosphorylated Smad2. Exposure to platelet releasates stirred at 1200 rpm increased the amount of phosphorylated Smad2 but not total Smad2.
Stirring enhances latent TGF-β1 activation. (A) Platelet releasate or (B) serum (200 μL) was placed in a glass aggregometer cuvette containing a metal stir bar and incubated at 37°C with and without stirring (1200 rpm) for the indicated time periods. Active TGF-β1 was measured directly in the ELISA, whereas total TGF-β1 (latent + active) was measured after activating latent TGF-β1 by treatment with acid. Stirring dramatically increased the amount of active TGF-β1 in both platelet releasates (3.5 ± 0.5 ng/mL, P < .001; n = 3) and sera (2.5 ± 0.6 ng/mL, P < .001; n = 4). Increasing the stirring speed enhanced latent TGF-β1 activation in both platelet releasates (C) and sera (D) when measured after 2 hours. Error bars represent SD. (E) MLECs were stimulated for 2 hours in serum-free medium with stirred or unstirred platelet releasates in the presence or absence of anti–TGF-β1 neutralizing antibody (α–TGF-β1; 2 μg/mL). Smad2 phosphorylation was detected by immunoblotting with a mAb specific for phosphorylated Smad2 (P-Smad2), and total Smad2 was detected with a polyclonal antibody that reacts with both phosphorylated and unphosphorylated Smad2. Exposure to platelet releasates stirred at 1200 rpm increased the amount of phosphorylated Smad2 but not total Smad2.
Shear force also enhances TGF-β1 activation
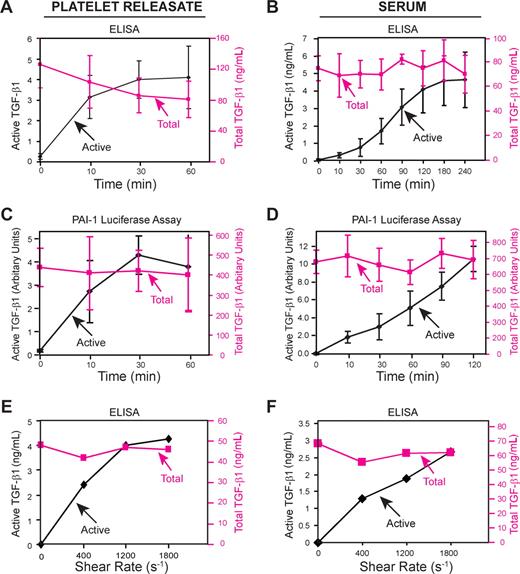
Because stirring produces complex and variable shear forces, depending on the location in the cuvette, we also subjected platelet releasates and sera to a uniform shear rate of 1800 s−1 in a cone and plate device at 37°C. TGF-β1 activity of platelet releasates increased progressively under shear, reaching a maximum of approximately 5% of total TGF-β1 as judged by ELISA (Figure 3A) and MLEC assays (Figure 3C; P < .001 for active TGF-β1 comparing 0 time to 60-minute samples for both assays; n = 3). In sharp contrast, without shear, active TGF-β1 constituted only approximately 0.2% of total, even after 2 hours at 37°C (data not shown). Activation of latent TGF-β1 in human sera under shear required 2 hours to reach a plateau, going from 0.2% of total TGF-β1 to a maximum of approximately 5% of total TGF-β1 as judged by ELISA (active: 4.1 ± 1.3 ng/mL; total: 76 ± 14 ng/mL; n = 6; Figure 3B). Similar results were obtained with the MLEC assay (Figure 3D). In accord with the stirring experiments, latent TGF-β1 activation increased with increasing shear rate (Figure 3E-F). Other methods that induce shear also enhanced latent TGF-β1 activation in platelet releasates including vortexing (5.5% ± 1.0% active after 30 minutes at 37°C; n = 3) and rapidly transferring the releasate back and forth between syringes joined together face to face by a plastic collar (∼ 10% active after 100 transfers at 37°C). The transfer between syringes was accomplished without exposing the releasate to air bubbles or an air-liquid interface so as to avoid any nonspecific oxidation or protein denaturing effects.
Shear enhances latent TGF-β1 activation. Platelet releasate or serum samples were added to a polystyrene plastic plate and subjected to a shear rate of 1800 s−1 with a rotating cone for the indicated time periods at 37°C. TGF-β1 activity was measured by ELISA (A,B) and by MLEC assay (C,D). Exposure to an increasing shear rate enhances latent TGF-β1 activation. (E) Platelet releasate or (F) serum was subjected to different shear rates for 2 hours in a cone and plate device at 37°C. Active and total TGF-β1 were measured by ELISA. Error bars represent SD.
Shear enhances latent TGF-β1 activation. Platelet releasate or serum samples were added to a polystyrene plastic plate and subjected to a shear rate of 1800 s−1 with a rotating cone for the indicated time periods at 37°C. TGF-β1 activity was measured by ELISA (A,B) and by MLEC assay (C,D). Exposure to an increasing shear rate enhances latent TGF-β1 activation. (E) Platelet releasate or (F) serum was subjected to different shear rates for 2 hours in a cone and plate device at 37°C. Active and total TGF-β1 were measured by ELISA. Error bars represent SD.
Thiol-disulfide exchange contributes to TGF-β1 activation
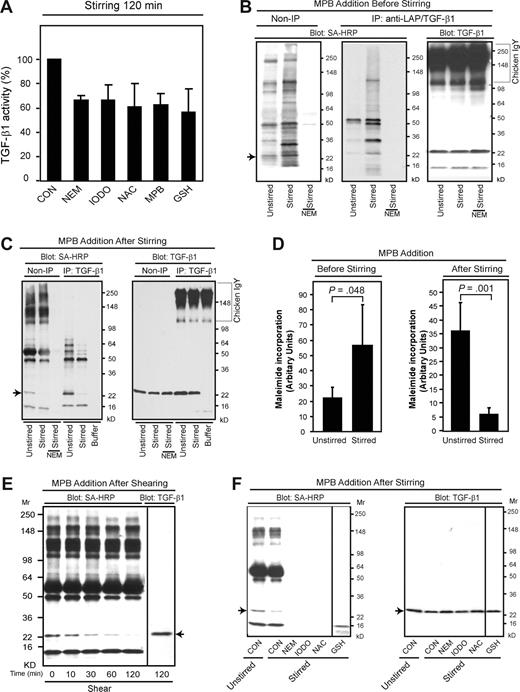
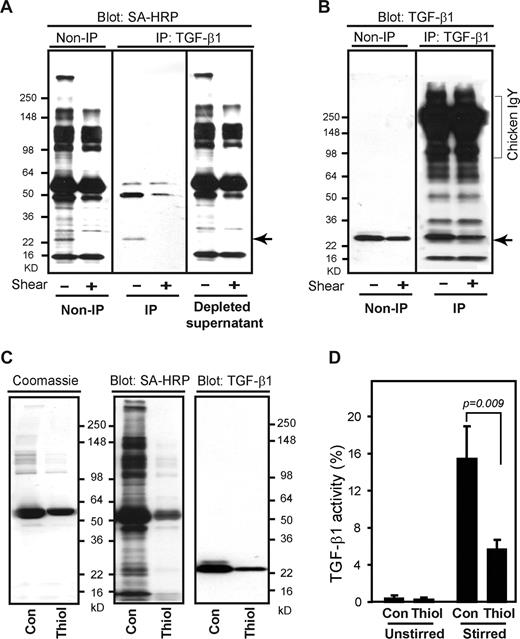
One potential molecular mechanism by which stirring and shear could affect latent TGF-β1 activation is through thiol-disulfide exchange, because mechanical force has been linked to the thiol-disulfide exchange underlying a number of biologic processes, including the control of von Willebrand factor multimerization,23 as well as exposure of intracellular protein thiols and control of thioredoxin catalytic activity in model systems.32,33 To assess whether a similar mechanism is involved in latent TGF-β1 activation, we tested the effect of adding various reagents that react with thiol groups to platelet releasates before stirring. All of these reagents reduced stirring-induced latent TGF-β1 activation by 30% to 40% (Figure 4A), suggesting that thiol-disulfide exchange contributes, at least in part, to the activation process. To identify the proteins that contain free thiols in platelet releasates, we added the sulfhydryl-reactive maleimide-biotin probe MPB to releasates immediately after they were prepared and analyzed the incorporation of MPB after 2 hours, with and without stirring. MPB labeled a variety of proteins, and the intensity of labeling was greater in the stirred samples (Figure 4B,D left panel). One of the MPB-labeled protein bands migrated identically to the TGF-β1 band (arrow), as judged both by immunoprecipitation using a combination of antibodies to LAP and TGF-β1 (Figure 4B middle panel) and by immunoblotting (Figure 4B right panel). Pretreating the releasates with the sulfhydryl reagent N-ethylmaleimide (NEM) prevented MPB labeling, even in the presence of stirring, supporting the specificity of the reaction for sulfhydryl groups (Figure 4B,C).
Thiol-disulfide exchange contributes to latent TGF-β1 activation. (A) Platelet releasates were stirred in the presence or absence of the indicated reagents for 120 minutes at 37°C and TGF-β1 activity was measured by ELISA. The concentrations used were as follows: N-ethylmaleimide (NEM) 1 mM, iodoacetamide (IODO) 1 mM, N-acetyl cysteine (NAC) 1 mM, MPB 100 μM, and glutathione (GSH) 200 μM. Stirring increased active TGF-β1 from approximately 0.2 to 5 ng/mL; total TGF-β1 was approximately 56 ng/mL. Data are expressed as percentage of the control value of active TGF-β1 in the absence of a thiol-reactive reagent (CON). Error bars represent SD. (B) MPB (100 μM) labeled a number of proteins in platelet releasates and the intensity of labeling increased when samples were stirred for 2 hours after adding the MPB. One of the labeled proteins was TGF-β1 itself (Mr, ∼ 25 kD) as judged by immunoprecipitation (middle panel) with a combination of anti-LAP and anti–TGF-β1 antibodies. (C) MPB labeling of the approximately 25-kD band corresponding to the migration of TGF-β1 (arrow), as well as some other proteins, was dramatically reduced when the MPB was added after 2 hours of stirring. Pretreatment with NEM (1 mM) prevented MPB labeling. (D) Densitometric quantification of biotin-MPB incorporation into the approximately 25-kD protein ( ) in panels B and C (left). Error bars represent SD. (E) Platelet releasates were subjected to shear at 1800 s−1 for the indicated time periods and then labeled with MPB (100 μM) for 2 hours at 37°C. MPB-labeled proteins in platelet releasates were detected with streptavidin-HRP. The sample sheared for 120 minutes from the same gel was also immunoblotted separately to identify TGF-β1. The vertical line separates the membranes reacted with streptavidin-HRP from the membrane reacted with the antibody to TGF-β1. (F) Thrombin-stimulated platelet releasates were incubated with the indicated thiol-reactive compounds followed by labeling with biotin-MPB (100 μM) for 2 hours. MPB-labeled proteins were detected with streptavidin-HRP (left panel) and immunoblotted to identify TGF-β1 (right panel). Vertical lines indicate deletion of intermediate lanes from the same gel.
) in panels B and C (left). Error bars represent SD. (E) Platelet releasates were subjected to shear at 1800 s−1 for the indicated time periods and then labeled with MPB (100 μM) for 2 hours at 37°C. MPB-labeled proteins in platelet releasates were detected with streptavidin-HRP. The sample sheared for 120 minutes from the same gel was also immunoblotted separately to identify TGF-β1. The vertical line separates the membranes reacted with streptavidin-HRP from the membrane reacted with the antibody to TGF-β1. (F) Thrombin-stimulated platelet releasates were incubated with the indicated thiol-reactive compounds followed by labeling with biotin-MPB (100 μM) for 2 hours. MPB-labeled proteins were detected with streptavidin-HRP (left panel) and immunoblotted to identify TGF-β1 (right panel). Vertical lines indicate deletion of intermediate lanes from the same gel.
Thiol-disulfide exchange contributes to latent TGF-β1 activation. (A) Platelet releasates were stirred in the presence or absence of the indicated reagents for 120 minutes at 37°C and TGF-β1 activity was measured by ELISA. The concentrations used were as follows: N-ethylmaleimide (NEM) 1 mM, iodoacetamide (IODO) 1 mM, N-acetyl cysteine (NAC) 1 mM, MPB 100 μM, and glutathione (GSH) 200 μM. Stirring increased active TGF-β1 from approximately 0.2 to 5 ng/mL; total TGF-β1 was approximately 56 ng/mL. Data are expressed as percentage of the control value of active TGF-β1 in the absence of a thiol-reactive reagent (CON). Error bars represent SD. (B) MPB (100 μM) labeled a number of proteins in platelet releasates and the intensity of labeling increased when samples were stirred for 2 hours after adding the MPB. One of the labeled proteins was TGF-β1 itself (Mr, ∼ 25 kD) as judged by immunoprecipitation (middle panel) with a combination of anti-LAP and anti–TGF-β1 antibodies. (C) MPB labeling of the approximately 25-kD band corresponding to the migration of TGF-β1 (arrow), as well as some other proteins, was dramatically reduced when the MPB was added after 2 hours of stirring. Pretreatment with NEM (1 mM) prevented MPB labeling. (D) Densitometric quantification of biotin-MPB incorporation into the approximately 25-kD protein ( ) in panels B and C (left). Error bars represent SD. (E) Platelet releasates were subjected to shear at 1800 s−1 for the indicated time periods and then labeled with MPB (100 μM) for 2 hours at 37°C. MPB-labeled proteins in platelet releasates were detected with streptavidin-HRP. The sample sheared for 120 minutes from the same gel was also immunoblotted separately to identify TGF-β1. The vertical line separates the membranes reacted with streptavidin-HRP from the membrane reacted with the antibody to TGF-β1. (F) Thrombin-stimulated platelet releasates were incubated with the indicated thiol-reactive compounds followed by labeling with biotin-MPB (100 μM) for 2 hours. MPB-labeled proteins were detected with streptavidin-HRP (left panel) and immunoblotted to identify TGF-β1 (right panel). Vertical lines indicate deletion of intermediate lanes from the same gel.
) in panels B and C (left). Error bars represent SD. (E) Platelet releasates were subjected to shear at 1800 s−1 for the indicated time periods and then labeled with MPB (100 μM) for 2 hours at 37°C. MPB-labeled proteins in platelet releasates were detected with streptavidin-HRP. The sample sheared for 120 minutes from the same gel was also immunoblotted separately to identify TGF-β1. The vertical line separates the membranes reacted with streptavidin-HRP from the membrane reacted with the antibody to TGF-β1. (F) Thrombin-stimulated platelet releasates were incubated with the indicated thiol-reactive compounds followed by labeling with biotin-MPB (100 μM) for 2 hours. MPB-labeled proteins were detected with streptavidin-HRP (left panel) and immunoblotted to identify TGF-β1 (right panel). Vertical lines indicate deletion of intermediate lanes from the same gel.
In sharp contrast, stirring the sample for 2 hours before adding MPB dramatically reduced MPB labeling of the Mr 25-kD band migrating at the same rate as TGF-β1 (Figure 4C left panel arrow and 4D right panel). A similar reduction in MPB labeling of the Mr 25-kD band migrating at the same rate as TGF-β1 was observed when platelet releasates were subjected to shear (1800 s−1) for increasing periods of time before adding MPB (Figure 4E), with virtually all of the labeling lost after 2 hours of shear (arrow). Thiol-reactive reagents that reduced latent TGF-β1 activation (Figure 4A) also inhibited MPB labeling (Figure 4F), supporting both the specificity of the MPB labeling and the association between thiol-disulfide exchange and latent TGF-β1 activation.
To further assess the identity of the MPB-labeled protein that migrated with an Mr identical to that of TGF-β1, MPB-labeled platelet releasates were immunoprecipitated with an anti–TGF-β1 antibody before and after being subjected to shear. The MPB-labeled band was immunoprecipitated by anti–TGF-β1 and was almost completely depleted from the supernatant (Figure 5A left panel). TGF-β1 could be detected by immunoblotting in both the platelet releasate and the samples immunoprecipitated with anti–TGF-β1 (Figure 5B right panel).
Depletion of thiol-containing proteins inhibits TGF-β1 activation. (A) Thrombin-stimulated platelet releasates were incubated without and with shear of 1800 s−1 for 2 hours and then labeled with MPB (100 μM) for 2 hours. MPB-labeled proteins in platelet releasates analyzed directly (non-IP) or after immunoprecipitation (IP) with an anti–TGF-β1. The MPB-labeled band at approximately 25 kD ( ) was immunoprecipitated by anti–TGF-β1 and depleted in the supernatant after IP. (B) The 25-kD band corresponded to TGF-β1 detected by immunoblotting of the entire releasate (non-IP) and the anti–TGF-β1 immunoprecipitate (IP: TGF-β1). Vertical lines indicate deletion of intermediate lanes. (C) Platelet releasates were passed through either a control Sepharose column (con) or a thiol-Sepharose column (thiol) and then labeled with MPB and analyzed by SDS-PAGE, followed by Coomassie brilliant blue staining (left panel), blotting with streptavidin-HRP (to detect MPB; middle panel), or blotting with an anti–TGF-β1 antibody (right panel). Note that the majority of MPB-labeled proteins were depleted by passage over the thiol-Sepharose column and that TGF-β1 was partially depleted. A Coomassie brilliant blue–stained gel demonstrated that the thiol-Sepharose column did not deplete most of the platelet releasate proteins. (D) Platelet releasates that were passed through either a control Sepharose column (con) or a thiol-Sepharose column (thiol) were stirred and assayed for TGF-β1 activation by ELISA. Note that depletion of thiol-containing proteins reduced the percentage of TGF-β1 that could be activated by stirring. Error bars represent SD.
) was immunoprecipitated by anti–TGF-β1 and depleted in the supernatant after IP. (B) The 25-kD band corresponded to TGF-β1 detected by immunoblotting of the entire releasate (non-IP) and the anti–TGF-β1 immunoprecipitate (IP: TGF-β1). Vertical lines indicate deletion of intermediate lanes. (C) Platelet releasates were passed through either a control Sepharose column (con) or a thiol-Sepharose column (thiol) and then labeled with MPB and analyzed by SDS-PAGE, followed by Coomassie brilliant blue staining (left panel), blotting with streptavidin-HRP (to detect MPB; middle panel), or blotting with an anti–TGF-β1 antibody (right panel). Note that the majority of MPB-labeled proteins were depleted by passage over the thiol-Sepharose column and that TGF-β1 was partially depleted. A Coomassie brilliant blue–stained gel demonstrated that the thiol-Sepharose column did not deplete most of the platelet releasate proteins. (D) Platelet releasates that were passed through either a control Sepharose column (con) or a thiol-Sepharose column (thiol) were stirred and assayed for TGF-β1 activation by ELISA. Note that depletion of thiol-containing proteins reduced the percentage of TGF-β1 that could be activated by stirring. Error bars represent SD.
Depletion of thiol-containing proteins inhibits TGF-β1 activation. (A) Thrombin-stimulated platelet releasates were incubated without and with shear of 1800 s−1 for 2 hours and then labeled with MPB (100 μM) for 2 hours. MPB-labeled proteins in platelet releasates analyzed directly (non-IP) or after immunoprecipitation (IP) with an anti–TGF-β1. The MPB-labeled band at approximately 25 kD ( ) was immunoprecipitated by anti–TGF-β1 and depleted in the supernatant after IP. (B) The 25-kD band corresponded to TGF-β1 detected by immunoblotting of the entire releasate (non-IP) and the anti–TGF-β1 immunoprecipitate (IP: TGF-β1). Vertical lines indicate deletion of intermediate lanes. (C) Platelet releasates were passed through either a control Sepharose column (con) or a thiol-Sepharose column (thiol) and then labeled with MPB and analyzed by SDS-PAGE, followed by Coomassie brilliant blue staining (left panel), blotting with streptavidin-HRP (to detect MPB; middle panel), or blotting with an anti–TGF-β1 antibody (right panel). Note that the majority of MPB-labeled proteins were depleted by passage over the thiol-Sepharose column and that TGF-β1 was partially depleted. A Coomassie brilliant blue–stained gel demonstrated that the thiol-Sepharose column did not deplete most of the platelet releasate proteins. (D) Platelet releasates that were passed through either a control Sepharose column (con) or a thiol-Sepharose column (thiol) were stirred and assayed for TGF-β1 activation by ELISA. Note that depletion of thiol-containing proteins reduced the percentage of TGF-β1 that could be activated by stirring. Error bars represent SD.
) was immunoprecipitated by anti–TGF-β1 and depleted in the supernatant after IP. (B) The 25-kD band corresponded to TGF-β1 detected by immunoblotting of the entire releasate (non-IP) and the anti–TGF-β1 immunoprecipitate (IP: TGF-β1). Vertical lines indicate deletion of intermediate lanes. (C) Platelet releasates were passed through either a control Sepharose column (con) or a thiol-Sepharose column (thiol) and then labeled with MPB and analyzed by SDS-PAGE, followed by Coomassie brilliant blue staining (left panel), blotting with streptavidin-HRP (to detect MPB; middle panel), or blotting with an anti–TGF-β1 antibody (right panel). Note that the majority of MPB-labeled proteins were depleted by passage over the thiol-Sepharose column and that TGF-β1 was partially depleted. A Coomassie brilliant blue–stained gel demonstrated that the thiol-Sepharose column did not deplete most of the platelet releasate proteins. (D) Platelet releasates that were passed through either a control Sepharose column (con) or a thiol-Sepharose column (thiol) were stirred and assayed for TGF-β1 activation by ELISA. Note that depletion of thiol-containing proteins reduced the percentage of TGF-β1 that could be activated by stirring. Error bars represent SD.
To obtain more detailed information on the role of proteins with thiol groups on latent TGF-β1 activation, we depleted platelet releasates of proteins with accessible thiol groups by passing the releasate through a thiol-Sepharose column. Compared with releasate passed through a control Sepharose column, this resulted in a 50% to 80% reduction in both total and thiol-containing proteins, as judged by Coomassie brilliant blue staining and MPB labeling followed by blotting with SA-HRP, respectively (Figure 5C). Total TGF-β1 was reduced by approximately 75% and the remaining TGF-β1 lost approximately 65% of its ability to be activated by stirring (Figure 5D; n = 3). Taken together, these findings suggest that many proteins in platelet releasates, including latent TGF-β1, contain free thiols that undergo disulfide bond formation during stirring or exposure to shear, and that thiol-disulfide exchange contributes, in part, to activation of latent TGF-β1.
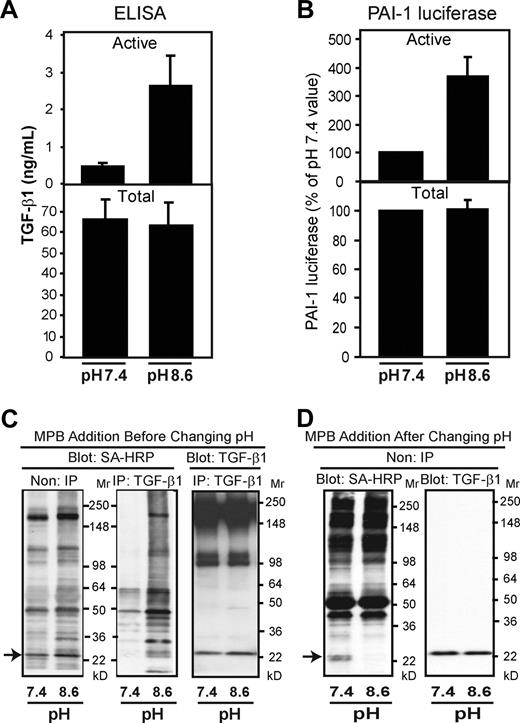
To explore further the potential contribution of thiol-disulfide exchange in the activation of platelet latent TGF-β1, we tested the effect of increasing the pH of the platelet releasate from 7.4 to 8.5, because thiol-disulfide exchange reactions are enhanced by the ionization of the sulfhydryl group produced by a more alkaline environment.34 Performing this shift in pH for 10 minutes at 37°C enhanced latent TGF-β1 activation approximately 5-fold, from 0.47 (± 0.1) to 2.6 (± 0.8) ng/mL (P = .001; n = 3), even without stirring or exposure to shear (Figure 6A). A similar effect was found when latent TGF-β1 activation was measured using the MLEC assay (Figure 6B). Increasing the pH from 7.4 to 8.6 in the presence of MPB also greatly increased MPB labeling of the releasate proteins, including the band that migrated at the same rate as TGF-β1 (Figure 6C). When MPB was added after the pH was increased for 10 minutes, however, the MPB labeling was markedly reduced (Figure 6D). Thus, increasing pH favors both thiol-disulfide exchange and latent TGF-β1 activation.
Increasing the platelet releasate pH from 7.4 to 8.6 enhances both latent TGF-β1 activation and thiol labeling. (A,B) Platelet releasate at pH 7.4 was adjusted to pH 8.6 by adding NaOH and incubated for 10 minutes at 37°C. Samples were neutralized to pH 7.4 by adding HCl. TGF-β1 activity was measured using both the ELISA (A) and MLEC assay (B). Error bars represent SD. (C,D) Platelet releasates at pH 7.4 and pH 8.6 were labeled with MPB (100 μM) before (C) or after (D) pH adjustment. Proteins labeled with MPB were detected with streptavidin (SA)–HRP before (non-IP) or after (IP) immunoprecipitation with an antibody to TGF-β1. TGF-β1 was detected with an antibody to TGF-β1. The amount of MPB incorporated into TGF-β1 increased when MPB was added before the pH shift, whereas less labeling occurred when MPB was added after the pH shift.
Increasing the platelet releasate pH from 7.4 to 8.6 enhances both latent TGF-β1 activation and thiol labeling. (A,B) Platelet releasate at pH 7.4 was adjusted to pH 8.6 by adding NaOH and incubated for 10 minutes at 37°C. Samples were neutralized to pH 7.4 by adding HCl. TGF-β1 activity was measured using both the ELISA (A) and MLEC assay (B). Error bars represent SD. (C,D) Platelet releasates at pH 7.4 and pH 8.6 were labeled with MPB (100 μM) before (C) or after (D) pH adjustment. Proteins labeled with MPB were detected with streptavidin (SA)–HRP before (non-IP) or after (IP) immunoprecipitation with an antibody to TGF-β1. TGF-β1 was detected with an antibody to TGF-β1. The amount of MPB incorporated into TGF-β1 increased when MPB was added before the pH shift, whereas less labeling occurred when MPB was added after the pH shift.
Latent TGF-β1 released by other cells can also be activated by stirring
To assess whether latent TGF-β1 produced by other cell types can similarly be activated by stirring, conditioned media obtained from human skin fibroblasts were subjected to stirring for 120 minutes and then both active and total TGF-β1 were measured. In 5 separate experiments performed on 2 different days, stirring caused more than a 30-fold increase in TGF-β1 activation when measured by ELISA, a value very similar to those observed with both platelet releasates and serum. Results from the MLEC assays were also similar (P < .01, day 1; P < .001, day 2), although the baseline values were somewhat higher (Table 1).
Effect of stirring on human fibroblast–conditioned medium
| . | Day 1 . | Day 2 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp 1 . | Exp 2 . | — . | Mean (SD) . | % Active (SD) . | Exp 1 . | Exp 2 . | Exp 3 . | Mean (SD) . | % Active (SD) . | |
| ELISA | ||||||||||
| Active TGF-β1, pg/mL | ||||||||||
| Unstirred | 3.0 | 4.0 | — | 3.5 (0.7) | 0.3 (0.1) | 0 | 0 | 0 | 0 | 0 |
| Stirred | 28 | 45 | — | 36 (12) | 9.7 (2.4) | 10 | 4 | 12 | 8.7 (4.0) | 4.2 (1.4) |
| Total TGF-β1, pg/mL | ||||||||||
| Unstirred | 952 | 948 | — | 950 (3) | — | 328 | 176 | 284 | 264 (76) | — |
| Stirred | 354 | 396 | — | 375 (36) | — | 256 | 140 | 208 | 200 (60) | — |
| MLEC assay | ||||||||||
| Active TGF-β1, arbitrary unit | ||||||||||
| Unstirred | 13.6 | 17.7 | 19.9 | 17.0 (3.2) | 4.0 (1.0) | 6.5 | 3.2 | 3.8 | 4.5 (1.7) | 1.8 (0.5) |
| Stirred | 28.0 | 29.0 | 27.0 | 28.0 (1.0) | 13 (1.0)† | 17.5 | 16.5 | 18.0 | 17.5 (0.9) | 17 (3)* |
| Total TGF-β1, arbitrary unit | ||||||||||
| Unstirred | 492 | 362 | 443 | 432 (65) | — | 261 | 219 | 218 | 233 (24) | — |
| Stirred | 226 | 198 | 191 | 205 (18) | — | 112 | 80 | 121 | 103 (21) | — |
| . | Day 1 . | Day 2 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp 1 . | Exp 2 . | — . | Mean (SD) . | % Active (SD) . | Exp 1 . | Exp 2 . | Exp 3 . | Mean (SD) . | % Active (SD) . | |
| ELISA | ||||||||||
| Active TGF-β1, pg/mL | ||||||||||
| Unstirred | 3.0 | 4.0 | — | 3.5 (0.7) | 0.3 (0.1) | 0 | 0 | 0 | 0 | 0 |
| Stirred | 28 | 45 | — | 36 (12) | 9.7 (2.4) | 10 | 4 | 12 | 8.7 (4.0) | 4.2 (1.4) |
| Total TGF-β1, pg/mL | ||||||||||
| Unstirred | 952 | 948 | — | 950 (3) | — | 328 | 176 | 284 | 264 (76) | — |
| Stirred | 354 | 396 | — | 375 (36) | — | 256 | 140 | 208 | 200 (60) | — |
| MLEC assay | ||||||||||
| Active TGF-β1, arbitrary unit | ||||||||||
| Unstirred | 13.6 | 17.7 | 19.9 | 17.0 (3.2) | 4.0 (1.0) | 6.5 | 3.2 | 3.8 | 4.5 (1.7) | 1.8 (0.5) |
| Stirred | 28.0 | 29.0 | 27.0 | 28.0 (1.0) | 13 (1.0)† | 17.5 | 16.5 | 18.0 | 17.5 (0.9) | 17 (3)* |
| Total TGF-β1, arbitrary unit | ||||||||||
| Unstirred | 492 | 362 | 443 | 432 (65) | — | 261 | 219 | 218 | 233 (24) | — |
| Stirred | 226 | 198 | 191 | 205 (18) | — | 112 | 80 | 121 | 103 (21) | — |
Exp indicates experiment; and —, not applicable.
P < .01 versus unstirred.
P < .001 versus unstirred.
TGF-β1 contained in small latent complexes cannot be activated by stirring
To assess whether stirring could activate latent TGF-β1 contained in isolated small latent complexes, we stirred recombinant human small latent complexes purified from Chinese hamster ovary (CHO) cells for 120 minutes and measured both active and total TGF-β1. Before stirring, approximately 1% of the total TGF-β1 was active, a value much higher than the values in platelet releasates. In 5 separate experiments, stirring increased TGF-β1 activity by only 30% when expressed as a percentage of total TGF-β1 (Table 2). Although the increase in activity was statistically significant (P = .002), it was less than the approximately 3000% increase found with both platelet releasates and sera (Figures 1,2). The purified small latent complexes differ from the large latent complexes in platelet releasates in not being covalently coupled to LTBP-1 and in being isolated from the other proteins present in platelet releasates and sera that might serve as activation cofactors.
Effect of stirring on recombinant human latent TGF-β1 (rhLTGF-β1)
| ELISA . | Experiment . | Mean (SD) . | % Active (SD) . | ||||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | |||
| Active TGF-β1, ng/mL | |||||||
| Unstirred | 0.55 | 0.38 | 0.35 | 0.41 | 0.39 | 0.41 (0.08) | 1.0 (0.08) |
| Stirred | 0.68 | 0.41 | 0.45 | 0.44 | 0.44 | 0.48 (0.11)* | 1.3 (0.16)† |
| Total TGF-β1, ng/mL | |||||||
| Unstirred | 58 | 37 | 37 | 40 | 37 | 41 (9) | |
| Stirred | 58 | 30 | 34 | 33 | 31 | 37 (12)* | |
| ELISA . | Experiment . | Mean (SD) . | % Active (SD) . | ||||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | |||
| Active TGF-β1, ng/mL | |||||||
| Unstirred | 0.55 | 0.38 | 0.35 | 0.41 | 0.39 | 0.41 (0.08) | 1.0 (0.08) |
| Stirred | 0.68 | 0.41 | 0.45 | 0.44 | 0.44 | 0.48 (0.11)* | 1.3 (0.16)† |
| Total TGF-β1, ng/mL | |||||||
| Unstirred | 58 | 37 | 37 | 40 | 37 | 41 (9) | |
| Stirred | 58 | 30 | 34 | 33 | 31 | 37 (12)* | |
P = .02 versus unstirred.
P = .002 versus unstirred.
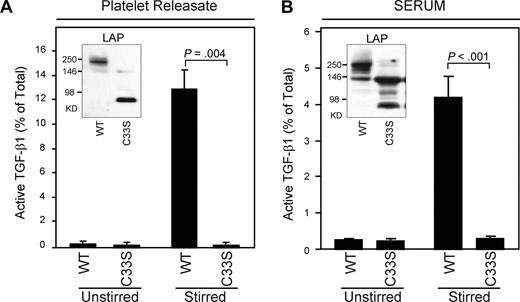
To assess the ability of small latent complexes to be activated by stirring in the presence of proteins found in platelet releasates and sera, we studied mice expressing an LAP containing a C33S mutation that prevents covalent coupling to LTBP-1. These mice have a phenotype similar to, but not quite as severe as, the phenotype of mice with targeted deletion of Tgfb1. The platelets of these mice contain small latent complexes rather than large latent complexes (Figure 7A inset). Stirring of platelet releasates and sera from the mutant mice produced very little or no activation of latent TGF-β1 despite the presence of the other proteins released from platelet α granules (Figure 7A,B).
TGF-β1 from mice expressing an LAP (C33S) mutation is present in small latent complexes rather than large latent complexes and cannot be activated by stirring. (A) Platelet releasates from wild-type (WT) or mutant (C33S) mice were either unstirred or stirred at 1200 rpm for 2 hours at 37°C, and active and total TGF-β1 were measured by ELISA (WT increased from 0.2% to 13%; mutant was 0.2% both before and after stirring; P < .005; n = 3). Platelet releasates were also immunoblotted with anti-LAP antibody. LAP migrated at an Mr compatible with large latent complex (Mr 270 kD) in WT mice but at an Mr compatible with small latent complex (Mr 80 kD) in C33S mutant mice (inset). (B) Sera from WT or C33S contained similar amounts of total TGF-β1 (WT mice: 63 ± 6 ng/mL; C33S mice: 67 ± 16 ng/mL). Mouse sera were either unstirred or stirred for 120 minutes at 37°C and TGF-β1 activity was measured by ELISA. TGF-β1 activity increased from 0.25% to 4.2% in WT mice but increased only from 0.23% to 0.3% in mutant mice (P < .001; n = 6). Sera were also immunoblotted with anti-LAP antibody and yielded results similar to those obtained with platelet releasate, but with several additional unidentified bands (inset). Error bars represent SD.
TGF-β1 from mice expressing an LAP (C33S) mutation is present in small latent complexes rather than large latent complexes and cannot be activated by stirring. (A) Platelet releasates from wild-type (WT) or mutant (C33S) mice were either unstirred or stirred at 1200 rpm for 2 hours at 37°C, and active and total TGF-β1 were measured by ELISA (WT increased from 0.2% to 13%; mutant was 0.2% both before and after stirring; P < .005; n = 3). Platelet releasates were also immunoblotted with anti-LAP antibody. LAP migrated at an Mr compatible with large latent complex (Mr 270 kD) in WT mice but at an Mr compatible with small latent complex (Mr 80 kD) in C33S mutant mice (inset). (B) Sera from WT or C33S contained similar amounts of total TGF-β1 (WT mice: 63 ± 6 ng/mL; C33S mice: 67 ± 16 ng/mL). Mouse sera were either unstirred or stirred for 120 minutes at 37°C and TGF-β1 activity was measured by ELISA. TGF-β1 activity increased from 0.25% to 4.2% in WT mice but increased only from 0.23% to 0.3% in mutant mice (P < .001; n = 6). Sera were also immunoblotted with anti-LAP antibody and yielded results similar to those obtained with platelet releasate, but with several additional unidentified bands (inset). Error bars represent SD.
Active TGF-β1 can be detected from platelet-rich thrombi formed in vivo
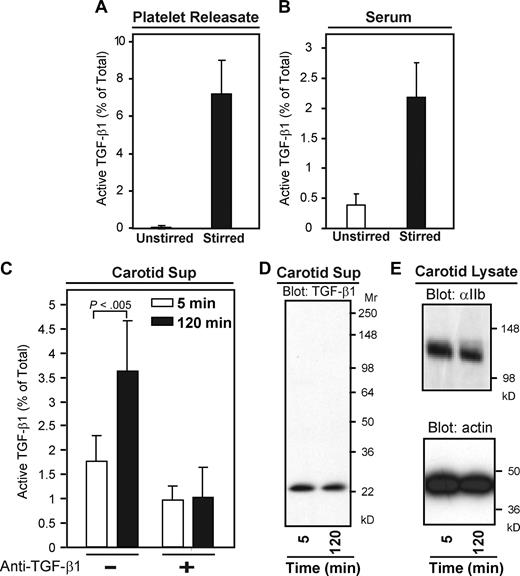
As with human platelet releasates, only 0.05% of TGF-β1 released from mouse platelets was active after 2 hours at 37°C without stirring, whereas stirring increased TGF-β1 activation to approximately 7% of total TGF-β1 (P < .005 compared with unstirred sample; n = 5; Figure 8A). Similarly, 0.3% of TGF-β1 was active in unstirred mouse sera after 2 hours, whereas approximately 2.5% of TGF-β1 was active after stirring at 37°C (Figure 8B, P < .001; n = 19).
Active TGF-β1 can be extracted from platelet-rich thrombi formed in vivo. (A) Mouse platelet releasate was stirred at 1200 rpm for 2 hours at 37°C and active and total TGF-β1 were measured by ELISA (P < 005; n = 5). (B) Mouse serum was stirred or unstirred for 120 minutes at 37°C and TGF-β1 activity was measured by ELISA (P < .001; n = 19). (C) Thrombosis was induced in the carotid arteries of C57Bl/6 mice by exposure to ferric chloride (8%) for 3 minutes. After thrombi formed (∼ 5 minutes), arterial segments (∼ 4 mm) were excised either 5 or 120 minutes thereafter. Thrombi were removed from the segments and dispersed in buffer (200 μL) on ice for 1 hour, after which the supernatants were collected by centrifugation. Total TGF-β1 was measured by ELISA and both active and total TGF-β1 were measured by the MLEC assay in the absence and presence of a TGF-β1 neutralizing antibody. Active TGF-β1 was detected in both samples, but the percentage of active TGF-β1 in the 120-minute thrombi was greater than in the 5-minute sample (P < .005; n = 6). Error bars represent SD. (D) Immunoblotting with an anti–TGF-β1 antibody confirmed the presence of TGF-β1 in the supernatants of 5- and 120-minute thrombi. The intensities corresponded to the amounts of total TGF-β1 found by ELISA (∼ 5 ng/mL at 5 minutes and ∼ 4 ng/mL at 120 minutes). (E) Immunoblotting of carotid lysate with anti-αIIb and actin antibodies also confirmed the presence of equal numbers of platelets in the thrombi.
Active TGF-β1 can be extracted from platelet-rich thrombi formed in vivo. (A) Mouse platelet releasate was stirred at 1200 rpm for 2 hours at 37°C and active and total TGF-β1 were measured by ELISA (P < 005; n = 5). (B) Mouse serum was stirred or unstirred for 120 minutes at 37°C and TGF-β1 activity was measured by ELISA (P < .001; n = 19). (C) Thrombosis was induced in the carotid arteries of C57Bl/6 mice by exposure to ferric chloride (8%) for 3 minutes. After thrombi formed (∼ 5 minutes), arterial segments (∼ 4 mm) were excised either 5 or 120 minutes thereafter. Thrombi were removed from the segments and dispersed in buffer (200 μL) on ice for 1 hour, after which the supernatants were collected by centrifugation. Total TGF-β1 was measured by ELISA and both active and total TGF-β1 were measured by the MLEC assay in the absence and presence of a TGF-β1 neutralizing antibody. Active TGF-β1 was detected in both samples, but the percentage of active TGF-β1 in the 120-minute thrombi was greater than in the 5-minute sample (P < .005; n = 6). Error bars represent SD. (D) Immunoblotting with an anti–TGF-β1 antibody confirmed the presence of TGF-β1 in the supernatants of 5- and 120-minute thrombi. The intensities corresponded to the amounts of total TGF-β1 found by ELISA (∼ 5 ng/mL at 5 minutes and ∼ 4 ng/mL at 120 minutes). (E) Immunoblotting of carotid lysate with anti-αIIb and actin antibodies also confirmed the presence of equal numbers of platelets in the thrombi.
To assess whether latent TGF-β1 becomes activated in vivo during thrombosis, we induced thrombi in the carotid arteries of C57Bl/6 mice by exposing them to ferric chloride (8%) for 3 minutes. All mice developed white, platelet-rich occlusive thrombi within 3 to 5 minutes. Segments (∼ 4 mm) of the arteries containing the thrombi were excised either 5 or 120 minutes after the thrombi formed, and the thrombi were removed from the segments and dispersed in buffer on ice for 1 hour. As measured by the ELISA, extracts from 5-minute thrombi contained 5.0 (± 2.0) ng/mL total TGF-β1 (n = 6) and extracts from 120-minute thrombi contained 4.0 (± 2.0) ng/mL total TGF-β1 (n = 6). Because the levels of active TGF-β1 were below the sensitivity of the ELISA, we used the more sensitive MLEC assay to measure active TGF-β1. Active TGF-β1 in 5-minute thrombi constituted 1.7% (± 0.5%) of the total TGF-β1 recovered (n = 6), and approximately 50% of this activity was inhibited by a TGF-β1 neutralizing antibody. In 120-minute thrombi, active TGF-β1 constituted 3.6% (± 1.0%) of the total recovered (P < .005 compared with 5 minutes value; n = 6) and almost 80% of the activity was neutralized by the anti–TGF-β1 antibody (Figure 8C). Based on the measured TGF-β1 activity, the volume of the supernatant, and the volume of plasma in the 4-mm arterial segments from which the thrombi were obtained (assuming a diameter of 0.7 mm), we calculated the intraluminal plasma level of active TGF-β1 as approximately 5.2 ng/mL in the 5-minute thrombi and approximately 14 ng/mL in the 120-minute thrombi. Immunoblotting confirmed the presence of TGF-β1 in both 5- and 120-minute samples, and the relative intensities correlated with the total TGF-β1 values (Figure 8D). Platelet-specific protein αIIb and actin in the carotid thrombi were also detected by immunoblotting as controls (Figure 8E).
Discussion
These results indicate that stirring or shear can dramatically enhance latent TGF-β1 activation after release from platelets or skin fibroblasts. The shear rate used in the platelet studies (1800 s−1) is within the range reported in normal arteries (1000-7000 s−1) and is considerably less than that reported in stenotic coronary arteries (up to 10 000 s−1).35 Our system differs from the in vivo situation, however, in that we applied shear continuously for 2 hours, whereas shear forces in vivo vary according to the cardiac cycle in complex patterns.
Stirring did not, however, activate latent TGF-β1 contained in small latent complexes either in isolation or in the presence of other proteins present in platelet releasates or serum. This suggests that covalent coupling of LAP to LTBP-1 may be necessary for stirring-induced latent TGF-β1 activation. The inability to activate the latent TGF-β1 in the platelet releasates and serum of the murine LAP C33S mutant with stirring is paralleled by a phenotype indicating a severe deficit in biologically active TGF-β1 (K.Y. et al 2008, manuscript in preparation). Thus, our data are consistent with a potential biologic role for shear-induced latent TGF-β1 activation.
To further assess the biologic relevance of our findings, we also conducted in vivo studies and found active TGF-β1 associated with platelet-rich thrombi in mice, with enhanced activation as a function of time after thrombus formation. These data are consistent with shear- and time-dependent activation of TGF-β1 released from platelets, but we recognize that other activation mechanisms may contribute to, or be responsible for, the observed activation. It is difficult to assess the shear in a blood vessel that is undergoing thrombosis because both the blood flow and blood vessel diameter are constantly changing. Our estimate of the intraluminal TGF-β1 concentration in the 120-minute platelet-rich thrombi (approximately 14 ng/mL) in the occluded vessel is more than 1000-fold higher than is required to initiate transcription of the PAI-1 gene in MLEC cells.20 Thus, in vivo platelet-rich thrombi generate biologically significant amounts of active TGF-β1.
Our observations are consistent with those of Blakytny et al, who briefly noted that shaking platelet suspensions greatly increased their levels of active TGF-β1 (reaching 1.8% of total); the authors speculated, however, that this was due to an effect on platelet activation rather than an effect on TGF-β1.15 In unpublished studies (J. Munger, I. Nunes, and D.B.R., 1997), we also observed that latent TGF-β1 obtained from conditioned medium from cultured cells could be activated to the same extent as with heating when rotated end-over-end in a test tube. Thus, shear-induced activation of latent TGF-β1 extends beyond platelet-derived TGF-β1 to include latent TGF-β1 produced by other cells.
Our data have potential implications for the role of platelet TGF-β1. It is likely that latent TGF-β1 released from platelets at the site of injury plays an important role in the physiologic process of wound healing, which involves an orchestrated set of responses by a variety of cell types, including fibroblasts that synthesize collagen.36 In pathologic states, however, such as embolization into the microcirculation of small platelet aggregates formed on an atherosclerotic plaque in a coronary artery or after vascular injury induced by percutaneous coronary intervention and stent placement,37 the release of latent TGF-β1 from platelets into a high shear environment could lead to a local fibrotic response, ultimately resulting in electrical instability and sudden cardiac arrest.38 This might provide a partial explanation for the observed long-term mortality benefits of short-term antiplatelet therapy during percutaneous coronary interventions.39 Because platelets may deposit in vascular beds throughout the body in different disease states, it is also possible that latent TGF-β1 released from platelets may contribute to tissue fibrosis associated with these disorders, as well as tissue-specific immune regulation.
It is also interesting to consider the possibility that intravascular shear-induced, local activation of latent TGF-β1 is a systemic analog of tissue-specific, traction-mediated activation of latent TGF-β1 controlled by interactions with both extracellular matrix and integrin receptors of contractile cells.5,–7,10,11 The fact that LTBP is required for both processes supports this suggestion. Additional data consistent with a shear-dependent systemic process comes from the study of Wang et al who detected elevations of active, but not total, TGF-β1 in the plasma of patients with coronary artery disease, with a gradient of increased values as a function of the number of vessels with more than 50% stenosis.40 The absolute values of plasma TGF-β1 that they reported were, however, much higher than those reported in other studies. TGF-β1 released from platelets during trauma or surgery might also contribute to the transient increases in plasma levels of PAI-1 that occur after these phenomena if the released latent TGF-β1 becomes activated intravascularly and systemically activates endothelial cells.41,,,,,–47 This would provide a valuable homeostatic link between platelet activation to arrest hemorrhage and transient inhibition of fibrinolysis to allow the early unopposed deposition of fibrin to secure hemostasis. In addition, the ability of shear force to activate latent TGF-β1 makes TGF-β1 a potential shear sensor in addition to being a cellular effector. Thus, for example, TGF-β1 may contribute to the vascular remodeling that occurs in response to changes in shear forces and maintains intravascular arterial shear within a limited range.35
Our data support a link between shear, thiol-disulfide exchange, and latent TGF-β1 activation. There are increasing numbers of examples of biologic phenomena that may be controlled by thiol-disulfide exchange, including some involving proteins released from, and on, platelets.48,,–51 Moreover, mechanical force has been implicated in model systems as contributing to or controlling exposure of thiol groups in proteins and thioredoxin-catalyzed thiol-disulfide exchange.23,32,33 Finally, our data may have implications for interpreting previous studies in which cells were subjected to shear in the presence of serum or under conditions in which the cells may have released latent TGF-β, because some of the observed effects may have been due to activation of latent TGF-β1 rather than a direct effect of the shear.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Ellinor Peerschke, Weill Cornell College of Medicine (New York, NY), for providing the cone and plate shear device; Dr Marketa Jirouskova for valuable suggestions; Jihong Li and Xiaorong Zhang for technical support; and Suzanne Rivera for administrative assistance.
This work was supported in part by grant 19278 from the National Heart, Lung and Blood Institute (B.S.C.), grant 034282 from the National Cancer Institute and Philip Morris USA (D.B.R.), a Clinical and Translational Science Award (CTSA; 5UL1RR024143) from the National Center for Research Resources (NIH), a New York City Community Trust (NYCT) Grant Award (New York, NY; J.A.), a Glorney-Raisbach Research Foundation Fellowship Award (New York, NY; N.B.), a Uehara Foundation Fellowship (Tokyo, Japan; K.Y.), and funds from Stony Brook University (Stony Brook, NY).
National Institutes of Health
Authorship
Contribution: J.A. designed and performed experiments, interpreted results, and wrote the paper; N.B. designed, performed, and interpreted select experiments; K.Y. generated the mice with the LAP C33S mutation and performed select experiments; C.A.J. performed the thrombosis model; D.B.R. generated the mice with the LAP C33S mutation, interpreted results, and edited the paper; and B.S.C. designed experiments, interpreted results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jasimuddin Ahamed, Laboratory of Blood and Vascular Biology, The Rockefeller University, 1230 York Avenue, New York, NY 10065; e-mail: jahamed@rockefeller.edu.