Hematopoiesis is a process by which mature blood cells are derived from an uncommitted hematopoietic stem cell (HSC). HSCs possess a unique property to both self-renew and to differentiate into any cell of the blood system.1 According to the classical model of hematopoiesis established by Weissman and colleagues, HSCs gradually lose their pluripotency and become more lineage-committed progenitors.2 The progenitors that are committed to the myeloid and the lymphoid lineages are referred as common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs), respectively. It has been assumed that all the cells of the myeloid lineage develop from CMPs and that all the cells of the lymphoid lineage are derived from CLPs. While this model might represent hematopoiesis under steady-state conditions, mounting evidence suggests that the differentiation pathways, particularly under stress conditions, are much more complex than earlier believed.
In recent years, several independent studies have documented that the cells of hematopoietic lineages undergo both “transdifferentiation” and “dedifferentiation” under specific conditions. Surprisingly, precursors and progenitors of the lymphoid lineage, such as CLPs, early thymic progenitors (ETPs), and CD19+ pro-B cells, have been reported to “transdifferentiate” into mature cells of the myeloid lineage.3 Similarly, through loss of function studies, it has been demonstrated that both mature T and B cells “dedifferentiate” into uncommitted progenitors with the potential of differentiating into cells of alternative lineages.3 Although all these studies have unequivocally demonstrated the broader developmental plasticity of lymphocyte lineage cells, the physiological significance of these phenomena is questionable, as most of these studies were conducted under what could be considered as “artificial” experimental conditions.
In an earlier study, Kincade et al reported that Toll-like receptor (TLR) stimulation in hematopoietic progenitor cells resulted in bypassing of normal differentiation steps by CMPs and drove CLPs to dendritic cells (DCs) in vitro.4 In this present study, Welner et al extended their previous findings and documented that the CLPs of wild-type mice infected with HSV-1 exhibit a bias to differentiate into DCs in vivo in a TLR9-dependent manner. Another interesting finding of the present study is that the B-cell development potential of CLPs was significantly reduced following HSV1 infection, while their natural killer (NK) cell differentiation potential remained unaffected.
In the paradigm of hematopoiesis (see figure), the ontogeny of dendritic cells is probably the most debated issue, and there has been a constant interest in deciphering the lineage identity of the precursors/progenitors of these professional antigen-presenting cells. To date, several major categories of DCs in mice have been identified, which are broadly classified as conventional DCs (cDCs), plasmacytoid DCs (pDCs), and migratory DCs (mDCs).5 One of the long-standing questions of DC biology has been recently resolved, at least to a certain extent, by the identification of a distinct common DC precursor (CDP) that can differentiate into both cDCs and pDCs in vivo.6 Thus, according to the current model of “dendropoiesis,” most of the DC subtypes might differentiate either from CDPs or from macrophage DC precursors (MDPs).6 In this regard, the work described by Welner et al gains much importance as they have highlighted that during viral infection, DCs preferentially differentiate from CLPs instead of CDPs. Of note, the present study did not reveal an increase of CDPs during viral infections. In keeping with their findings, an earlier notion. that a common pathway might exist between DCs and B cells has been supported.7,8
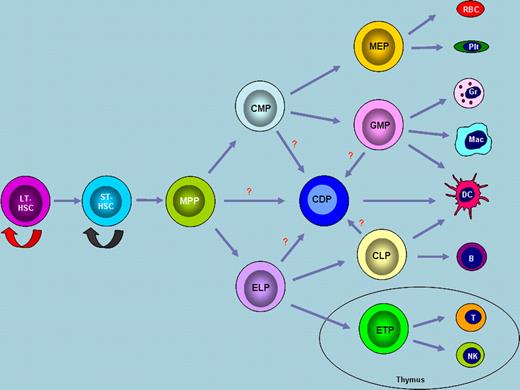
The paradigm of hematopoiesis. During hematopoiesis, long-term hematopoietic stem cells (LT-HSCs) differentiate into short-term hematopoietic stem cells (ST-HSCs) and subsequently into multipotent progenitors (MPPs). LT-HSCs have unlimited abilities to self-renew, while ST-HSCs have limited self-renewal capacity. MPPs differentiate into CMPs and ELPs. Erythroid and myeloid lineage cells are differentiated from CMPs, whereas cells of the lymphoid lineage are derived from ELPs. Dendritic cells are developed from recently identified CDPs. Although this model represents the most widely accepted view, alternative theories exist regarding the commitment of lineage-restricted progenitors. Abbreviations: MEP, megakaryocyte erythrocyte progenitor; GMP, granulocyte monocyte progenitor; CLP, common lymphoid progenitor; ETP, early thymic progenitor; RBC, red blood cell; Plt, platelet; Gr, granulocyte; Mac, macrophage; DC, dendritic cell; B, B cell; T, T cell; NK, natural killer cell.
The paradigm of hematopoiesis. During hematopoiesis, long-term hematopoietic stem cells (LT-HSCs) differentiate into short-term hematopoietic stem cells (ST-HSCs) and subsequently into multipotent progenitors (MPPs). LT-HSCs have unlimited abilities to self-renew, while ST-HSCs have limited self-renewal capacity. MPPs differentiate into CMPs and ELPs. Erythroid and myeloid lineage cells are differentiated from CMPs, whereas cells of the lymphoid lineage are derived from ELPs. Dendritic cells are developed from recently identified CDPs. Although this model represents the most widely accepted view, alternative theories exist regarding the commitment of lineage-restricted progenitors. Abbreviations: MEP, megakaryocyte erythrocyte progenitor; GMP, granulocyte monocyte progenitor; CLP, common lymphoid progenitor; ETP, early thymic progenitor; RBC, red blood cell; Plt, platelet; Gr, granulocyte; Mac, macrophage; DC, dendritic cell; B, B cell; T, T cell; NK, natural killer cell.
However, it should be emphasized here that the observations of Welner et al might not reflect lineage differentiation pathways during steady-state conditions and might be triggered only under specific inflammatory settings. Nevertheless, the present study raises many intriguing questions: Why would DCs differentiate from CLPs instead of CDPs during infections? Why would TLR ligation suppress B-cell differentiation but not NK-cell differentiation capacity of CLPs? What are the genetic factors favoring the “transdifferentiation” of CLPs into DCs? What are the functional properties of DCs that are differentiated from CLPs? How can external stimuli reprogram the genetic control of hematopoiesis? How economical would it be, for the hematopoietic system, to reroute lineage differentiation pathways?
One more interesting highlight from the present study is the involvement of TLRs in hematopoiesis. The current study by Welner et al confirms and extends the previous findings of the same group that TLRs play a critical role in directing the hematopoietic cell fates under inflammatory conditions. It is very interesting to learn that the functions of TLRs extend beyond its well known role in regulating the innate immune responses during infections. It is also surprising to observe that TLRs violate normal paradigm of hematopoiesis and have the ability to reroute the normal differentiation pathways.
To date, the in vivo significance of “transdifferentiation” and relevance of such a phenomenon has not been appreciated, even though the concept has been known for some time. The present study by Welner et al warrants further consideration of this possibility and sheds light on the epigenetic control of hematopoiesis.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal