Abstract
Targeting mechanisms of neutrophil elastase (NE) and other luminal proteins stored in myeloperoxidase (MPO)–positive secretory lysosomes/primary granules of neutrophils are unknown. These granules contain an integral membrane protein, CD63, with an adaptor protein-3–dependent granule delivery system. Therefore, we hypothesized that CD63 cooperates in granule delivery of the precursor of NE (proNE). Supporting this hypothesis, an association was demonstrated between CD63 and proNE upon coexpression in COS cells. This also involved augmented cellular retention of proNE requiring intact large extracellular loop of CD63. Furthermore, depletion of CD63 in promyelocytic HL-60 cells with RNA interference or a CD63 mutant caused reduction of cellular NE. However, the proNE steady-state level was similar to wild type in CD63-depleted clones, making it feasible to examine possible effects of CD63 on NE trafficking. Thus, depletion of CD63 led to reduced processing of proNE into mature NE and reduced constitutive secretion. Furthermore, CD63-depleted cells showed a lack of morphologically normal granules, but contained MPO-positive cytoplasmic vacuoles with a lack of proNE and NE. Collectively, our data suggest that granule proteins may cooperate in targeting; CD63 can be involved in ER or Golgi export, cellular retention, and granule targeting of proNE before storage as mature NE.
Introduction
Hematopoietic cells play a critical role in host defense, for which they are equipped with secretory lysosomes or lysosome-related organelles that can release cell-specific cytolytic proteins by regulated secretion.1,2 The secretory lysosomes/primary granules of neutrophils are furnished with an array of proteins that includes hematopoietic serine proteases along with myeloperoxidase (MPO), other microbicidal proteins, and specific transmembrane proteins.3,4 The precise functions of the hematopoietic serine proteases—neutrophil elastase (NE), cathepsin G, proteinase 3, and azurocidin—are not fully known, but all 4 are thought to be important in the innate immunity function provided by neutrophils.5,6 These proteases are synthesized as transient proforms that become catalytically active (except for azurocidin) by removal of an N-terminal propeptide after granule targeting.7,8
Lysosome hydrolases and granzymes use a mannose-6-phosphate (MP) signal and binding to an MP receptor for targeting,9,10 but the signals for the targeting of hematopoietic serine proteases are not yet known. Cargo proteins can be transported to secretory lysosomes independent of the MP system,11 as is illustrated by the fact that in I-cell disease, neutrophils and other cells have a normal content of hydrolases despite a lack of MP synthesis.12 The delivery of a soluble protein might occur through the assistance of a cooperating transmembrane partner that establishes contact with adaptor proteins (APs) to recruit the transport system necessary for targeting. The adaptor protein AP-3 is known to have a role in bringing transmembrane protein cargo to the lysosome from the trans-Golgi network and the late endosome.13,14 The AP-3 machinery is also important in NE targeting, as is demonstrated by the fact that AP-3 deficiency in canine cyclic hematopoiesis prevents trafficking of NE to granules,15 and by the reduction of intracellular neutrophil elastase that accompanies the AP-3 mutation in patients with the Hermansky-Pudlak type 2 syndrome.16 The lysosome-associated membrane proteins LAMP-1 and LAMP-2 are absent from neutrophil secretory lysosomes,17,18 which contain the tetraspanin CD63 (also known as LAMP-3)19 as a major integral membrane protein.20 Therefore, we hypothesized that NE precursor (proNE) is routed to granules through the protein-delivery pathway of CD63, which recruits AP-3 adaptor complex through the C-terminal lysosomal targeting motif GYEVM.21 Thus, cooperation with CD63 was thought to facilitate the secretory lysosome targeting of proNE.
To corroborate our hypothesis, we examined whether CD63 and proNE interacted upon coexpression in COS cells. Furthermore, we examined how CD63 depletion affected proNE trafficking. We selectively suppressed CD63 with RNA interference and a dominant-negative inhibition in promyelocytic HL-60 cells, which synthesize proteins such as NE for storage in primary granules. Our findings suggest that CD63 may be involved in the cellular trafficking and retention of proNE for storage in the primary granules of neutrophils.
Methods
Materials
Antibodies.
The following antibodies were used: mouse monoclonal human NE-antibody, RDI-ELASTabm-203 (Research Diagnostics, Flanders, NJ); rabbit anti–human NE (Calbiochem, San Diego, CA); rabbit anti–human MPO22 ; mouse monoclonal human LAMP-3 (CD63) antibody, MX-49.129.5, and mouse monoclonal human actin antibody, SC-8432 (Santa Cruz Biotechnology, San Diego, CA); rabbit anti-cproNE against the C-terminal propeptide CPHPRDPDPASRTH of human NE19 ; and murine monoclonal anti-GFP no. 1814460 (Roche, Basel, Switzerland).
Buffers.
Cell lysis buffer, radioimmune precipitation buffer (RIPA), and electrophoresis sample buffer were prepared as previously described.19
Expression vectors and transfections.
The following CD63 expression plasmids were kindly donated by Dr Sato of the University of Tokyo: pRK-GFP-CD63, pRK-GFP-CD63ΔLEL lacking the large extracellular loop (Val106-Lys186), and pRK-GFP-CD63ΔC12 lacking the C-terminal cytoplasmic domain (Leu227-Met238).23 The NE expression plasmid used was the full-length human NE, including the C-terminal and N-terminal propeptides (Figure 1A).4,19
COS cells were seeded in 10-cm2 Petri dishes and transfected the next day using Polyfect (Qiagen, Valencia, CA), and cell lysates were examined after 24 hours or reseeded overnight for fluorescence microscopy.
CD63 was suppressed by RNA interference using stable transfection of HL-60 cells by electroporation19 with pSuppressorNeo-CD63 (kindly obtained from Dr Codina, Winston-Salem, NC)24 and a nonrelevant scrambled siRNA vector, IMG 800-6 (Imgenex, San Diego, CA), as control. The CD63 of HL-60 cells was also suppressed by dominant-negative inhibition with pCDNA3.1-Hygro-FLAG-CD63AEVM (kindly provided by Dr Caplan, Yale University, New Haven, CT), which carries C-terminal GAEVM instead of the normal GYEVM, disrupting the lysosomal targeting.21,25
Real-time polymerase chain reaction
Samples of mRNA and cDNA were prepared using RNeasy and Omniscript RT kits (Qiagen) according to manufacturer's instructions. The analyses were performed using the TaqMan Gene Expression Assay kit (Applied Biosystems, Foster City, CA) and a 7000-sequence detection system. The primers used were assay ID Hs00156390_m1 (CD63), Hs00357734_m1 (NE), and Hs99999901_s1 (18S), purchased from Applied Biosystems. Data were normalized against the endogenous control (18S) and compared with wild-type cells according to the ΔΔCT method. The same data were also normalized against other endogenous controls (B2-microglobulin and GAPDH) with similar results (not shown).
Radiolabeling
Cells were radiolabeled with 30 μCi (1.11 MBq)/mL [35S]methionine/[35S]cysteine (ICN Pharmaceuticals, Santa Ana, CA), as previously described.26,27 To follow protein processing, the radiolabel was chased in RPMI supplemented with 10% fetal bovine serum (FBS). At indicated time points, 20 × 106 cells and incubation medium were withdrawn, extracted in RIPA and protease inhibitors (Complete; Roche Diagnostics, Mannheim, Germany), and examined by immunoprecipitation (IP).
IP and IP/Western
Cells (2 × 106/mL) were incubated with IP-lysis buffer (250 mM NaCl, 20 mM Na-phosphate, 30 mM Na-pyrophosphate [pH 7.0], 5 mM EDTA, 0.1 mM Na3Vo4, 10 mM NaF, and 0.1% NP40) to preserve the complex. IP was performed in a mixture of antiserum, 50 mg/mL protein A–Sepharose, and 2% (vol/vol) protein G–agarose, and rotated overnight. A pellet was recovered by centrifugation, washed 3 times with RIPA, dissolved in electrophoresis sample buffer, boiled for 5 minutes, and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) in a 10% to 20% Tris-glycine gel (Novex; Invitrogen, Frederick, MD). The gel was exposed to X-ray film at −80°C for 14 days. Densitometry was performed in a Molecular Imager FX (BioRad, Hercules, CA). For IP/Western, SDS-PAGE was followed by transfer to a PVDF membrane, incubated with antibody and then horseradish peroxidase–conjugated secondary antibody. The Western blots were developed using an enhanced chemiluminescence (ECL) kit (GE Healthcare, Little Chalfont, United Kingdom). All steps were performed at 4°C. For protein visualization, gels were stained with silver nitrate.
Immunofluorescence microscopy
Unpermeabilized and permeabilized COS cells and HL-60 cells were stained as previously described.19
Immunoelectron microscopy
Cells were fixed for 24 hours in 4% paraformaldehyde in 0.1 M PHEM buffer (60 mM PIPES, 25 mM HEPES, 2 mM MgCl2, and 10 mM EGTA, pH 6.9) and then processed for ultrathin cryosectioning as previously described.28 The cryosections were incubated at room temperature with anti-MPO or anti-NE and then incubated with 10-nm protein A–conjugated colloidal gold (EM Lab, Utrecht University, The Netherlands) as previously described.28 After immunolabeling, the cryosections were embedded in a mixture of methylcellulose and uranyl acetate and examined with a Philips CM10 electron microscope (Eindhoven, The Netherlands). For controls, primary antibodies were replaced with nonrelevant rabbit antiserum.
Flow cytometry
Cells were incubated for 15 minutes in blocking buffer before the addition of primary antibody (mouse anti-NE; dilution, 1:600) for 15 minutes. After washing with blocking buffer, secondary antibody (goat antimouse Alexa Fluor 488 F(ab)2 fragment [dilution, 1:600] or goat antirabbit Alexa Fluor 488 F(ab)2 fragment [dilution, 1:600]) was added for 15 minutes. For intracellular staining, cells were fixed in 1% CellFIX (Becton Dickinson [BD], Stockholm, Sweden) and permeabilizing blocking buffer, incubated with primary antibody for 2 hours, washed with permeabilizing blocking buffer, and incubated with secondary antibody for 1 hour as previously described.19 Cells were washed twice with permeabilizing blocking buffer before analysis on a FACSCalibur using CellQuest software (BD), saving up to 50 000 events.
Results
ProNE and CD63 interact when coexpressed in COS cells
A schematic diagram of the constructs used is shown in Figure 1A. The construct NE, coding for preproNE, is shown with signal peptide, N-terminal dipropeptide, mature enzyme sequence, and C-terminal propeptide. N-terminal fusions of CD63 with GFP were used for transfection of COS cells to gain evidence to support the hypothesis of an interaction between proNE and CD63. Anti-cproNE antibody detected proNE by both IP and immunoblotting (Tapper et al19 and data not shown). Anti-NE antibody detected both NE and proNE by IP, and detected NE (eg, in neutrophil extracts; data not shown) but not proNE by immunoblotting. In experiments repeated at least 3 times, immunoblotting with anti-cproNE antibody of cell lysates (Figure 1B top) showed 31-kDa proNE and slightly smaller processing forms with intact C-termini, allowing detection with anti-cproNE. No proNE was detected in lysates of untransfected cells used as negative control (Figure 1B WT). IP/Western data demonstrated coprecipitation of proNE and GFP-CD63 (Figure 1B). Thus, we conclude that anti-GFP pulled down proNE, and anti-cproNE pulled down GFP-CD63, indicating that these proteins were present in the same complex. Similar results were obtained for the CD63 mutant GFP-CD63ΔLEL lacking the large extracellular loop (Figure 1B). We ruled out an interaction between proNE and GFP itself after cotransfection and examination with IP/Western (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
CD63 and proNE form a complex. (A) A schematic diagram of the constructs used. The NE (preproNE) construct is shown with signal peptide, N-terminal dipropeptide (SE), mature enzyme sequence, and C-terminal propeptide. The full-length CD63 and the CD63 mutants; CD63ΔLEL, lacking the large extracellular loop (LEL), CD63Δ12 with deleted C-terminal lysosomal targeting motif, and CD63 dom neg (dominant-negative inhibition) with a single amino acid change in the C-terminal sorting motif are shown. N-terminal fusions of the CD63 variants with GFP were used for transfection of COS cells. The CD63 siRNA construct used is also shown with the complementary mRNA region forming a hairpin and marking the corresponding mRNA target region in the protein. (B) COS cells were transfected with plasmids carrying GFP-CD63 (CD63), NE, or GFP-CD63ΔLEL, as indicated. Cell lysates were examined by immunoblotting with anti-actin, anti-GFP (for detection of GFP-CD63 fusion proteins), and anti-cproNE, showing comparable expression of the plasmids. IP/Western was performed to examine coprecipitation between CD63 and proNE. Both GFP-CD63 and GFP-CD63ΔLEL coprecipitated proNE, as judged by IP with anti-GFP followed by Western blotting with anti-cproNE. In addition, proNE coprecipitated the GFP-CD63 and GFP-CD63ΔLEL, as judged by IP with anti-cproNE followed by immunoblotting with anti-GFP. (C) COS cells transfected as indicated were incubated for 24 hours with [35S]methionine/[35S]cysteine to achieve steady-state radiolabeling of proteins and then examined by IP and fluorography. A major radiolabeled 31-kDa proNE band was detected in cells expressing proNE or proNE plus GFP-CD63 (marked to the right with proNE). Upon coexpression, IP with anti-cproNE copurified proteins migrating as GFP-CD63 (●) or GFP-CD63ΔLEL (▲), and IP with anti-GFP copurified proteins migrating as proNE. In addition, GFP-CD63ΔLEL (▲) copurified proNE and vice versa. These data confirm complex formation between proNE and GFP-CD63 or GFP-CD63ΔLEL. The 85-kDa band of the supernatant corresponds to a covalent complex between proNE and α1-proteinase inhibitor.
CD63 and proNE form a complex. (A) A schematic diagram of the constructs used. The NE (preproNE) construct is shown with signal peptide, N-terminal dipropeptide (SE), mature enzyme sequence, and C-terminal propeptide. The full-length CD63 and the CD63 mutants; CD63ΔLEL, lacking the large extracellular loop (LEL), CD63Δ12 with deleted C-terminal lysosomal targeting motif, and CD63 dom neg (dominant-negative inhibition) with a single amino acid change in the C-terminal sorting motif are shown. N-terminal fusions of the CD63 variants with GFP were used for transfection of COS cells. The CD63 siRNA construct used is also shown with the complementary mRNA region forming a hairpin and marking the corresponding mRNA target region in the protein. (B) COS cells were transfected with plasmids carrying GFP-CD63 (CD63), NE, or GFP-CD63ΔLEL, as indicated. Cell lysates were examined by immunoblotting with anti-actin, anti-GFP (for detection of GFP-CD63 fusion proteins), and anti-cproNE, showing comparable expression of the plasmids. IP/Western was performed to examine coprecipitation between CD63 and proNE. Both GFP-CD63 and GFP-CD63ΔLEL coprecipitated proNE, as judged by IP with anti-GFP followed by Western blotting with anti-cproNE. In addition, proNE coprecipitated the GFP-CD63 and GFP-CD63ΔLEL, as judged by IP with anti-cproNE followed by immunoblotting with anti-GFP. (C) COS cells transfected as indicated were incubated for 24 hours with [35S]methionine/[35S]cysteine to achieve steady-state radiolabeling of proteins and then examined by IP and fluorography. A major radiolabeled 31-kDa proNE band was detected in cells expressing proNE or proNE plus GFP-CD63 (marked to the right with proNE). Upon coexpression, IP with anti-cproNE copurified proteins migrating as GFP-CD63 (●) or GFP-CD63ΔLEL (▲), and IP with anti-GFP copurified proteins migrating as proNE. In addition, GFP-CD63ΔLEL (▲) copurified proNE and vice versa. These data confirm complex formation between proNE and GFP-CD63 or GFP-CD63ΔLEL. The 85-kDa band of the supernatant corresponds to a covalent complex between proNE and α1-proteinase inhibitor.
Probing of the anti-NE and anti-GFP immunoprecipitates against actin, which is abundant in COS cells, gave negative results, lending specificity to our data (not shown).
To further elucidate the interactions between proNE and CD63, biosynthetic radiolabeling was used to allow direct examination of the proteins by IP and fluorography. Transfected cells were incubated for 24 hours with [35S]methionine/[35S]cysteine to achieve steady-state radiolabeling of proteins. A major radiolabeled 31-kDa proNE band was detected in cells expressing NE or NE plus GFP-CD63 (Figure 1C). Upon coexpression, IP with anti-NE copurified proteins migrating as the CD63 variants, and IP with anti-GFP copurified proteins migrating as proNE. These data also confirm an interaction between proNE and GFP-CD63. In addition, GFP-CD63ΔLEL copurified proNE and vice versa, confirming that a complex was formed with this mutant, too (Figure 1C).
RIPA and cell lysis buffer were comparable in cell extraction. Cell lysis buffer with 0.05 or 0.25 M NaCl gave identical results, suggesting that ionic interaction was unimportant for complex formation (data not shown).
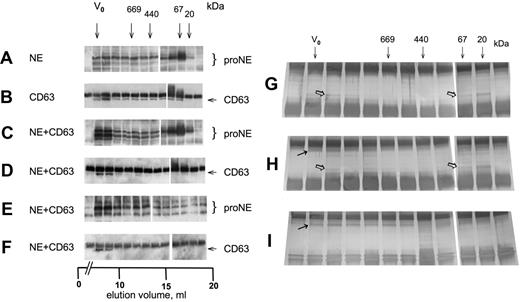
On gel filtration, proNE was eluted as a monomer and to a minor part as a high-molecular-weight complex, as judged by IP/Western (Figure 2A). GFP-CD63 showed a broad elution profile (Figure 2B) consistent with its assumed presence in a tetraspanin web.29,30 Both proNE and GFP-CD63 of cell lysate coexpressing these proteins eluted to a large extent at the void volume, which was consistent with their presence in high-molecular-weight complexes (Figure 2C,D). To characterize the complexes formed upon coexpression of NE and CD63, these were pulled down by immunoprecipitation with anti-GFP (Figure 2E). The complexes contained cproNE eluted at the void volume with trailing in lower-molecular-weight fractions. Similarly, the reverse experiment demonstrated that the complex pulled down with anti-cproNE contained CD63 detected at the void volume only (Figure 2F). IP with anti-cproNE carried out on cells expressing NE and followed by SDS-PAGE and silver staining visualized protein corresponding both to proNE monomer and partly to proNE complex of high molecular weight (Figure 2G). Similarly, IP with anti-cproNE of cells coexpressing NE and GFP-CD63 visualized silver-stained protein corresponding to monomer and complex (Figure 2H). Furthermore, IP copurified a protein corresponding to GFP-CD63 eluted with complexed proNE but not with monomeric proNE, indicating the presence of complexes containing both proteins. IP with anti-GFP showed a broad elution profile of a stained protein corresponding to GFP-CD63, similar to the finding in Figure 2B, but copurification of proNE was barely visible (Figure 2I).
CD63 and proNE form high-molecular-weight complexes. (A-F) Cell lysates in RIPA from COS cells transfected with NE, GFP-CD63 (CD63), and NE plus GFP-CD63 as indicated were separated on a Superose 6 column eluted with RIPA. The fractions were analyzed by IP/Western as follows: (A) anti-cproNE/anti-cproNE, (B) anti-GFP/anti-GFP, (C) anti-cproNE/anti-cproNE, (D) anti-GFP/anti-GFP, (E) anti-GFP/anti-cproNE, and (F) anti-cproNE/anti-GFP. The samples were split (empty column) as there were too many for a single gel. (G-I) Cell lysates were examined by IP, SDS-PAGE, and silver staining as indicated. (G) IP with anti-cproNE of cells expressing NE showed stained protein corresponding both to proNE monomer and proNE complex ( ). (H) IP with anti-cproNE of cells coexpressing NE and GFP-CD63 also showed stained protein corresponding to proNE monomer and complex (
). (H) IP with anti-cproNE of cells coexpressing NE and GFP-CD63 also showed stained protein corresponding to proNE monomer and complex ( ), and copurification of a protein corresponding to GFP-CD63 (→). (I) IP with anti-GFP showed a broad elution profile of a stained protein corresponding to GFP-CD63 (→) as in panel B, but copurification of proNE was barely visible. Arrows show the void volume (V0) and elution volumes for thyroglobulin (669 kDa), ferritin (440 kDa), bovine serum albumin (67 kDa), and trypsin inhibitor (20 kDa).
), and copurification of a protein corresponding to GFP-CD63 (→). (I) IP with anti-GFP showed a broad elution profile of a stained protein corresponding to GFP-CD63 (→) as in panel B, but copurification of proNE was barely visible. Arrows show the void volume (V0) and elution volumes for thyroglobulin (669 kDa), ferritin (440 kDa), bovine serum albumin (67 kDa), and trypsin inhibitor (20 kDa).
CD63 and proNE form high-molecular-weight complexes. (A-F) Cell lysates in RIPA from COS cells transfected with NE, GFP-CD63 (CD63), and NE plus GFP-CD63 as indicated were separated on a Superose 6 column eluted with RIPA. The fractions were analyzed by IP/Western as follows: (A) anti-cproNE/anti-cproNE, (B) anti-GFP/anti-GFP, (C) anti-cproNE/anti-cproNE, (D) anti-GFP/anti-GFP, (E) anti-GFP/anti-cproNE, and (F) anti-cproNE/anti-GFP. The samples were split (empty column) as there were too many for a single gel. (G-I) Cell lysates were examined by IP, SDS-PAGE, and silver staining as indicated. (G) IP with anti-cproNE of cells expressing NE showed stained protein corresponding both to proNE monomer and proNE complex ( ). (H) IP with anti-cproNE of cells coexpressing NE and GFP-CD63 also showed stained protein corresponding to proNE monomer and complex (
). (H) IP with anti-cproNE of cells coexpressing NE and GFP-CD63 also showed stained protein corresponding to proNE monomer and complex ( ), and copurification of a protein corresponding to GFP-CD63 (→). (I) IP with anti-GFP showed a broad elution profile of a stained protein corresponding to GFP-CD63 (→) as in panel B, but copurification of proNE was barely visible. Arrows show the void volume (V0) and elution volumes for thyroglobulin (669 kDa), ferritin (440 kDa), bovine serum albumin (67 kDa), and trypsin inhibitor (20 kDa).
), and copurification of a protein corresponding to GFP-CD63 (→). (I) IP with anti-GFP showed a broad elution profile of a stained protein corresponding to GFP-CD63 (→) as in panel B, but copurification of proNE was barely visible. Arrows show the void volume (V0) and elution volumes for thyroglobulin (669 kDa), ferritin (440 kDa), bovine serum albumin (67 kDa), and trypsin inhibitor (20 kDa).
CD63 increases intracellular retention of proNE in COS cells
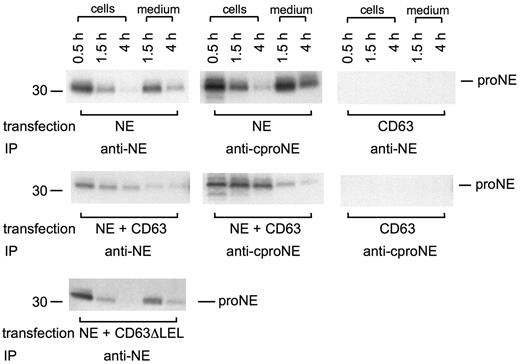
The interaction between proNE and CD63 was further examined using biosynthetic pulse-chase radiolabeling and IP to follow the fate of newly synthesized protein. Transfections were as in Figure 1B to guarantee equal expression during coexpression. ProNE was detected equally by IP with anti-NE or anti-cproNE. ProNE remained unprocessed during a 4-hour chase of the radiolabel and was constitutively secreted (Figure 3). However, coexpression with GFP-CD63 led to increased intracellular retention of proNE (Figure 3), suggesting cooperation between the 2 proteins. In contrast to wild-type GFP-CD63, cotransfection with GFP-CD63ΔLEL did not result in cellular retention of coexpressed proNE, indicating that the large extracellular loop of CD63 plays some role (Figure 3). Furthermore, the results argue against overexpression being a cause of cellular retention; then proNE would have been retained when coexpressed also with GFP-CD63ΔLEL.
Intracellular retention of proNE is augmented by CD63. COS cells transfected with NE, GFP-CD63, NE plus GFP-CD63, and NE plus GFP-CD63ΔLEL (lacking the large extracellular loop) were subject to biosynthetic radiolabeling for 0.5 hours followed by chase of the radiolabel for 1.5 and 4 hours. Cells were extracted in RIPA and immunoprecipitated with anti-NE or anti-cproNE followed by SDS-PAGE and fluorography. Both anti-NE and anti-cproNE detect proNE in cells expressing NE and NE plus GFP-CD63 in a similar manner in both cells and medium. Cells transfected with GFP-CD63 did not show any signal after IP with anti-NE or anti-cproNE. Coexpression of NE and GFP-CD63 but not NE and GFP-CD63ΔLEL led to increased intracellular retention of proNE.
Intracellular retention of proNE is augmented by CD63. COS cells transfected with NE, GFP-CD63, NE plus GFP-CD63, and NE plus GFP-CD63ΔLEL (lacking the large extracellular loop) were subject to biosynthetic radiolabeling for 0.5 hours followed by chase of the radiolabel for 1.5 and 4 hours. Cells were extracted in RIPA and immunoprecipitated with anti-NE or anti-cproNE followed by SDS-PAGE and fluorography. Both anti-NE and anti-cproNE detect proNE in cells expressing NE and NE plus GFP-CD63 in a similar manner in both cells and medium. Cells transfected with GFP-CD63 did not show any signal after IP with anti-NE or anti-cproNE. Coexpression of NE and GFP-CD63 but not NE and GFP-CD63ΔLEL led to increased intracellular retention of proNE.
ProNE and CD63 colocalize in COS cells
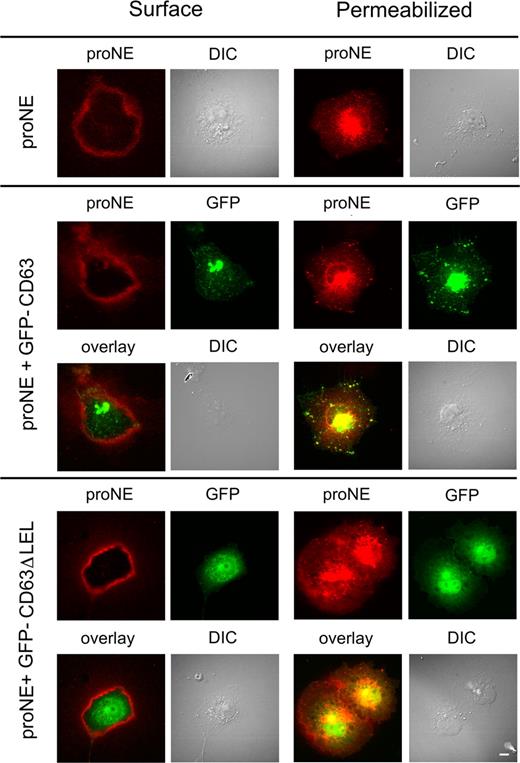
Immunofluorescence microscopy was used to seek further support for an association between proNE and CD63 and their intracellular retention (Figure 4). Unpermeabilized live and permeabilized COS cells were examined after staining with anti-cproNE. Surface staining of proNE was visible in live cells of all transfectants. Cells coexpressing NE and GFP-CD63 showed both proNE and GFP in the perinuclear/Golgi region and, more importantly, colocalization of proNE and GFP in cytoplasmic organelles, corresponding to lysosomes. In contrast, colocalization of proNE and GFP-CD63ΔLEL in cytoplasmic organelles was not seen in cells coexpressing these proteins. The colocalization of proNE and GFP-CD63 observed in cytoplasmic organelles was consistent not only with an interaction between these proteins (Figures 1,2) but also with their cellular retention (Figure 3). Furthermore, the lack of colocalization observed between proNE and GFP-CD63ΔLEL in these organelles was consistent with deficient retention (Figure 3). Therefore, the interaction observed between proNE and GFP-CD63ΔLEL (Figure 1) may occur in the secretory pathway prior to cytoplasmic organelles in the endoplasmic reticulum (ER) or the Golgi; the overlay shows staining of proNE and GFP in the perinuclear region (Figure 4). This interaction may be of low efficiency, allowing proNE to escape by constitutive secretion (Figure 3). In addition, these proteins did not colocalize in cytoplasmic organelles in cells coexpressing NE and GFP-CD63Δ12 (lacking the C-terminal lysosomal sorting motif; data not shown). Furthermore, GFP-CD63Δ12 transfectants lacked a GFP signal in cytoplasmic organelles, which was consistent with this mutant's retention in the ER or plasma-membrane transfer.
Colocalization of proNE and GFP-CD63 in COS cells. COS cells were transfected with plasmids carrying NE, NE plus GFP-CD63, and NE plus GFP-CD63ΔLEL (lacking the large extracellular loop) as indicated. Unpermeabilized live cells (surface) and permeabilized cells were examined by immunofluorescence microscopy after staining with anti-proNE. Surface staining of proNE is visible in all transfectants, but appears to be strongest in cells coexpressing GFP-CD63ΔLEL. In permeabilized cells expressing NE or NE plus GFP-CD63, proNE staining is concentrated in the perinuclear space and cytoplasmic organelles; the double transfectant shows colocalization of proNE and GFP. In contrast, cells coexpressing NE plus GFP-CD63ΔLEL show much lower colocalization of proNE and GFP. Images were recorded on an Eclipse TE 300 (Nikon Nordic AB, Solna, Sweden) inverted-fluorescence microscope equipped with a C4742-95 cooled CCD camera (Hamamatsu; Photonics; Norden AB, Solna, Sweden) using a plan Apochromat 60×/1.40 oil objective and ImageProPlus version 4.5.0.29 (Media Cybemetics, Bethesda, MD). Scale bar represents 10 μm.
Colocalization of proNE and GFP-CD63 in COS cells. COS cells were transfected with plasmids carrying NE, NE plus GFP-CD63, and NE plus GFP-CD63ΔLEL (lacking the large extracellular loop) as indicated. Unpermeabilized live cells (surface) and permeabilized cells were examined by immunofluorescence microscopy after staining with anti-proNE. Surface staining of proNE is visible in all transfectants, but appears to be strongest in cells coexpressing GFP-CD63ΔLEL. In permeabilized cells expressing NE or NE plus GFP-CD63, proNE staining is concentrated in the perinuclear space and cytoplasmic organelles; the double transfectant shows colocalization of proNE and GFP. In contrast, cells coexpressing NE plus GFP-CD63ΔLEL show much lower colocalization of proNE and GFP. Images were recorded on an Eclipse TE 300 (Nikon Nordic AB, Solna, Sweden) inverted-fluorescence microscope equipped with a C4742-95 cooled CCD camera (Hamamatsu; Photonics; Norden AB, Solna, Sweden) using a plan Apochromat 60×/1.40 oil objective and ImageProPlus version 4.5.0.29 (Media Cybemetics, Bethesda, MD). Scale bar represents 10 μm.
The participation of serglycin in NE targeting has been reported.31,32 Therefore, the possible presence of proteoglycans was examined, as previously described by Lemansky et al.32 COS cells coexpressing NE and GFP-CD63 were incubated with radioactive sulfate, immunoprecipitated with anti-cproNE, and subjected to SDS-PAGE followed by fluorography. ProNE did not pull down any 35S-labeled macromolecules (data not shown), indicating that proteoglycan was not present.
CD63 is depleted by siRNA or dominant-negative CD63 inhibition in HL-60 cells
The COS cell system is artificial and may still be affected by overexpression. Therefore, the role of CD63 in NE sorting was also examined in HL-60 cells, by suppression of CD63 by siRNA or a dominant-negative mutant. Transfection with pSuppressorNeo-CD63 expressing CD63 RNA interference produced 11 geneticin-resistant clones, of which 5 were shown to knock down CD63 (CD63 siRNA) (Figure 5A). One geneticin-resistant clone that did not knock down CD63 was used as negative control (CD63 siRNA mock). Control data with scrambled siRNA are given in Figure S2. To obtain dominant-negative CD63, cells were transfected with pCDNA3.1 Hygro-CD63AEVM-Flag. This mutant produced 8 hygromycin-resistant clones that knocked down CD63 (CD63 dom neg). A pcDNA3.1-Hygro mock transfectant (CD63 dom neg mock) was used as negative control (Figure 5A). The CD63AEVM mutant has a single amino acid mutation in the C-terminal lysosomal sorting sequence that decreases intracellular CD63 and plasma membrane sorting.21 Although not formally proved, CD63AEVM might produce nonfunctional complexes that are likely to be degraded or become transferred to the plasma membrane.
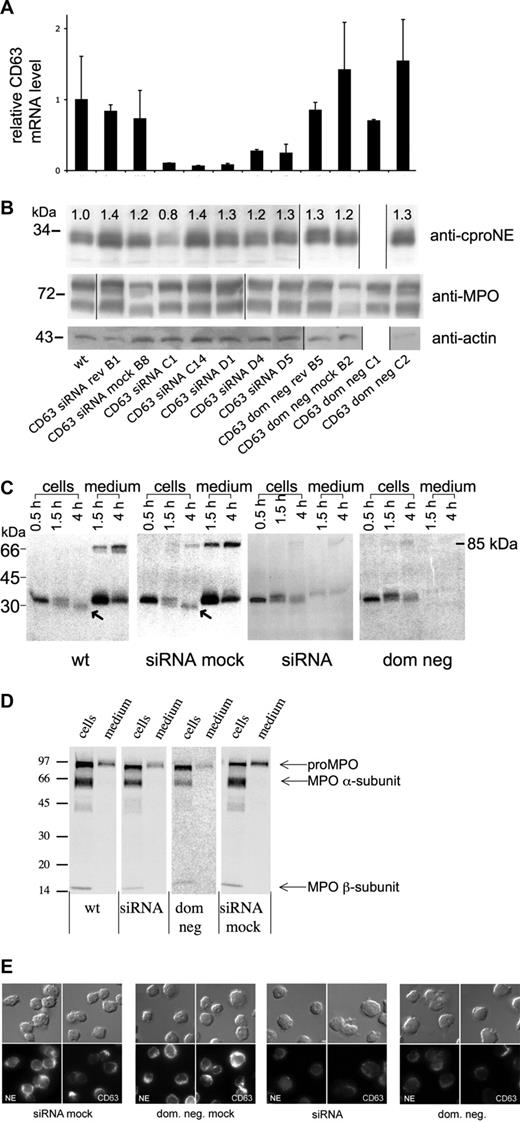
Suppression of CD63 in HL-60 cells does not affect the level of proNE but diminishes the amount of mature NE and inhibits proNE processing and secretion. (A) CD63 of HL-60 cells is suppressed by CD63 siRNA—real-time PCR. The figure shows the relative mRNA levels for CD63 compared with wild-type cells (wt) with 18S mRNA as an internal control. The clones shown are CD63 siRNA mock, CD63 siRNA rev (reverted CD63 siRNA clone), CD63 siRNA (5 clones), CD63 dom neg mock, CD63 dom neg rev (reverted CD63 dom neg clone), and CD63 dom neg (2 clones). Data presented are mean plus or minus SD of 1 representative experiment of 3 similar experiments, each done in triplicate from 1 run of real-time PCR, and calculated according to the ΔΔCT method. The mean suppression of all CD63 siRNA clones was 6.5-fold for CD63. CD63 dom neg clones showed no significant suppression of CD63 mRNA. Notice the normalization of CD63 mRNA levels in the reverted CD63 siRNA clone. (B) ProNE of HL-60 cells is not diminished by CD63 depletion—Western blotting. The panel shows immunoblotting with anti-proNE, anti-MPO, and anti-actin of cell lysates from the same clones as in panel A, except for lack of clone CD63 dom neg C1. The numbers on top represent the relative densitometry values normalized for wild-type (wt) cells. The amount of proNE detected in 4 of 5 different siRNA clones and 1 CD63 dom neg clone was similar to wild-type cells. Actin is shown as control. Molecular weights in kilodaltons are shown to the left. (C) NE processing and secretion are inhibited—biosynthetic radiolabeling. Wild-type (wt), CD63 siRNA mock (siRNA mock), CD63 siRNA (siRNA), and CD63 dom neg (dom neg) HL-60 cells were radiolabeled (pulse) for 30 minutes, followed by chase of the radiolabel for up to 4 hours. The 85-kDa band of the supernatant corresponds to a covalent complex between proNE and α1-proteinase inhibitor. Different processing forms are seen in the figure: 31-kDa unglycosylated apoproNE, glycosylated 34-kDa proNE, and mature 29-kDa NE (marked with arrows). Wild-type and CD63 siRNA mock cells display the processing into mature NE forms and constitutive secretion of proNE. CD63 siRNA and CD63 dom neg cells lack both processing of proNE and constitutive secretion. The numbers to the left are molecular mass standards. (D) Processing and secretion of MPO in wild-type (wt), CD63 siRNA (siRNA), CD63 dom neg (dom neg), and CD63 siRNA mock-transfected (siRNA mock) cells was investigated by overnight radiolabeling and IP with anti-MPO and then examined by SDS-PAGE followed by fluorography. Processing of pro-MPO into mature α- and β-subunits was also observed after CD63 suppression by CD63 siRNA or CD63 dom neg. (E) NE of HL-60 cells is diminished by CD63 depletion—immunofluorescence microscopy. The panel shows CD63 and NE staining (using anti-NE antibody) observed in CD63 siRNA, CD63 siRNA mock, CD63 dom neg, and CD63 dom neg mock HL-60 cells. Depletion of CD63 by siRNA or a dom neg mutant was associated with down-regulation of NE.
Suppression of CD63 in HL-60 cells does not affect the level of proNE but diminishes the amount of mature NE and inhibits proNE processing and secretion. (A) CD63 of HL-60 cells is suppressed by CD63 siRNA—real-time PCR. The figure shows the relative mRNA levels for CD63 compared with wild-type cells (wt) with 18S mRNA as an internal control. The clones shown are CD63 siRNA mock, CD63 siRNA rev (reverted CD63 siRNA clone), CD63 siRNA (5 clones), CD63 dom neg mock, CD63 dom neg rev (reverted CD63 dom neg clone), and CD63 dom neg (2 clones). Data presented are mean plus or minus SD of 1 representative experiment of 3 similar experiments, each done in triplicate from 1 run of real-time PCR, and calculated according to the ΔΔCT method. The mean suppression of all CD63 siRNA clones was 6.5-fold for CD63. CD63 dom neg clones showed no significant suppression of CD63 mRNA. Notice the normalization of CD63 mRNA levels in the reverted CD63 siRNA clone. (B) ProNE of HL-60 cells is not diminished by CD63 depletion—Western blotting. The panel shows immunoblotting with anti-proNE, anti-MPO, and anti-actin of cell lysates from the same clones as in panel A, except for lack of clone CD63 dom neg C1. The numbers on top represent the relative densitometry values normalized for wild-type (wt) cells. The amount of proNE detected in 4 of 5 different siRNA clones and 1 CD63 dom neg clone was similar to wild-type cells. Actin is shown as control. Molecular weights in kilodaltons are shown to the left. (C) NE processing and secretion are inhibited—biosynthetic radiolabeling. Wild-type (wt), CD63 siRNA mock (siRNA mock), CD63 siRNA (siRNA), and CD63 dom neg (dom neg) HL-60 cells were radiolabeled (pulse) for 30 minutes, followed by chase of the radiolabel for up to 4 hours. The 85-kDa band of the supernatant corresponds to a covalent complex between proNE and α1-proteinase inhibitor. Different processing forms are seen in the figure: 31-kDa unglycosylated apoproNE, glycosylated 34-kDa proNE, and mature 29-kDa NE (marked with arrows). Wild-type and CD63 siRNA mock cells display the processing into mature NE forms and constitutive secretion of proNE. CD63 siRNA and CD63 dom neg cells lack both processing of proNE and constitutive secretion. The numbers to the left are molecular mass standards. (D) Processing and secretion of MPO in wild-type (wt), CD63 siRNA (siRNA), CD63 dom neg (dom neg), and CD63 siRNA mock-transfected (siRNA mock) cells was investigated by overnight radiolabeling and IP with anti-MPO and then examined by SDS-PAGE followed by fluorography. Processing of pro-MPO into mature α- and β-subunits was also observed after CD63 suppression by CD63 siRNA or CD63 dom neg. (E) NE of HL-60 cells is diminished by CD63 depletion—immunofluorescence microscopy. The panel shows CD63 and NE staining (using anti-NE antibody) observed in CD63 siRNA, CD63 siRNA mock, CD63 dom neg, and CD63 dom neg mock HL-60 cells. Depletion of CD63 by siRNA or a dom neg mutant was associated with down-regulation of NE.
In the 5 clones investigated, CD63 siRNA expression showed a mean of 6.5-fold (the range was from 16-fold to 3.7-fold) suppression of CD63 mRNA compared with wild-type cells, CD63 siRNA mock, or a reverted CD63siRNA clone (Figure 5A). The CD63 mRNA levels of 5 cell clones with scrambled siRNA control vector did not differ from wild-type cells (Figure S2A). In contrast to CD63 siRNA clones, the CD63 dom neg clones showed no significant suppression of CD63 mRNA; real-time polymerase chain reaction (PCR) for CD63 mRNA detected both CD63 wild-type and CD63 dom neg mRNA. Thus, CD63 siRNA but not CD63 dom neg showed suppression of CD63 mRNA. After 8 weeks in culture without geneticin, the CD63 siRNA and CD63 dom neg clones examined recovered full expression of CD63 and NE and were indistinguishable from wild-type and mock-transfected cells (data not shown). The growth rate for CD63-depleted HL-60 cells was slightly lower than that for mock-transfected and wild-type cells. The level of NE mRNA varied between both scrambled siRNA control clones (11%-177%; Figure S2B) and wild-type clones (not shown). NE mRNA of CD63 siRNA-depleted cells was reduced to 25% of the level in wild-type cells (Figure S3). All mRNA data given are normalized against the housekeeping gene 18s. Two additional housekeeping genes have been used with similar results (GAPDH and β2-microglobulin).
Depletion of CD63 interferes with proNE processing, blocks secretion, and reduces mature NE in HL-60 cells
Despite downmodulation of NE mRNA by CD63 siRNA (Figure S3), the proNE protein steady-state levels were unaffected, as detected by immunoblotting using the anti-cproNE antibody. Thus, quantification by densitometry showed the amount of proNE in 4 of 5 different siRNA clones and 1 CD63 dom neg clone examined to be similar to or higher than that in wild-type cells (Figure 5B). Furthermore, the amount of proNE in CD63 siRNA mock, CD63 dom neg mock, and reverted clones was also similar to that in wild-type cells (Figure 5B; Figure S2C,E). The steady-state level of another neutrophil granule protein, MPO, was not affected by CD63 depletion (Figure 5B).
To determine whether CD63 depletion affected NE processing and secretion, biosynthetic radiolabeling was performed and the progress of newly synthesized proNE was followed (Figure 5C). During chase of the radiolabel for 4 hours, the 34-kDA proNE of wild-type and siRNA mock cells was either constitutively secreted or processed into a 29-kDa component corresponding to mature NE (Figure 5C). This 29-kDa component was not immunoprecipitated with the anti-cproNE antibody, indicating that at least processing with deletion of the C-terminal propeptide had taken place (data not shown). Despite a decrease in NE mRNA (Figure S3), proNE biosynthesis of CD63-depleted cells was similar to that of wild-type/mock cells as judged by 0.5-hour pulse radiolabeling (Figure 5C). As for processing, proNE of CD63-depleted cells showed both 31-kDa unglycosylated apoproNE and glycosylated 34-kDa proNE, but, in contrast to wild-type/mock cells, lacked further processing (Figure 5C). Radiolabeled proNE in wild-type/mock cells disappeared within 4 hours of chase by constitutive secretion and conversion into 29-kDa product. Radiolabeled proNE of CD63-depleted cells, in contrast, was not subject to constitutive secretion during chase but rather decreased by intracellular degradation. To examine constitutive secretion, a ratio between intracellular and extracellular proNE was determined by densitometry. The following ratios were found (based on the mean value of 2 clones of each condition): wild-type cells, 0.3; CD63 siRNA mock, 0.4; CD63 dom neg mock, 0.2; CD63 siRNA, 3.6; and CD63 dom neg, 8.8. These data show that constitutive secretion of newly synthesized proNE was 60% to 80% in control cells but only 1.4% in CD63-depleted cells. NE biosynthesis and processing in cells transfected with scrambled siRNA control vector did not differ from wild-type cells (Figure S2F). Support for degradation in CD63-depleted cells was obtained by densitometric readings showing a ratio of 1.5 between 1.5- and 4-hour chase of the radiolabel for CD63 siRNA (mean of 4 clones) and 1.9 for CD63 dom neg (mean of 2 clones). Importantly, the steady-state level of proNE was similar in cells depleted by CD63 siRNA or dominant-negative CD63, lending further specificity to the results. Thus, despite degradation, the lack of constitutive secretion in CD63-depleted cells explains a similar steady-state level of proNE in these cells and wild-type/mock cells (Figure 5B). Our findings are consistent with the existence of a block in proNE export at or proximal to the Golgi induced by CD63 suppression.
The influence of CD63 on the biosynthesis and processing of MPO, another major granule constituent of neutrophils,33 was also examined. Steady-state radiolabeling was achieved by overnight incubation of cells. Typical proteolytic processing of 90-kDa proMPO into mature 59-kDa α and 13.5-kDa β subunits was observed for cells expressing wild-type CD63, CD63 siRNA, CD63 siRNA mock, or CD63 dom neg (Figure 5D). Densitometric readings showed the ratios between proMPO and the α subunit to be 1.3, 1.4, 2.1, and 1.0 for these 4 variants. The corresponding ratios between intracellular proMPO and extracellular proMPO (constitutive secretion) were 3.8, 3.7, 5.6, and 1.8, respectively. These data show that depletion of CD63 did not affect MPO processing and secretion.
Cellular CD63 and NE were visualized by immunostaining with anti-NE antibody, which reacts with both proNE and NE. A CD63 signal and an NE signal were observed both at the cell surface of wild-type HL-60 cells and inside them (data not shown). Figure 5E shows that depletion of CD63 by CD63 siRNA or CD63 dom neg was accompanied by a decreased NE signal. Mock-transfected cells and scrambled siRNA control clones showed expression similar to wild-type cells (Figure 5E; Figure S2D).
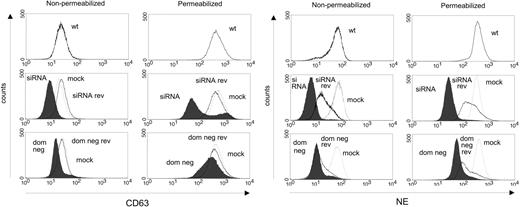
The effects of CD63 depletion were confirmed by flow cytometry (Figure 6; Figure S2E). Nonpermeabilized live cells were stained to measure the cell-surface expression of NE. In live cells lacking functional CD63, reduced expression of NE was observed. Both CD63 siRNA and CD63 dom neg mutants had lower levels of NE than mock-transfected controls. The NE expression of the mock-transfected cells was comparable to wild-type cells. Cells were also fixed and permeabilized to show intracellular NE. The expression of NE was again lower in CD63 siRNA and CD63 dom neg mutants than in mock-transfected cells, which had a similar expression to wild-type cells. In conclusion, transfection with CD63 siRNA or CD63 dom neg resulted in a major depletion of CD63 expression, accompanied by a decreased NE signal. In contrast, the level of proNE was not affected by CD63 depletion (Figure 5B). Because anti-cproNE, used for immunoblotting, detects only proNE, whereas anti-NE, used for immunostaining and flow cytometry, detects both proNE and mature NE, the results indicate that mature NE was reduced by CD63 depletion, whereas proNE was not.
NE of HL-60 cells is diminished by CD63 depletion—flow cytometry. Wild-type (wt), CD63 siRNA, CD63 siRNA mock (mock; thin line), reverted CD63 siRNA clone (siRNA rev; thick line), CD63 dom neg, CD63 dom neg mock (mock; thin line), and reverted CD63 dom neg (dom neg rev; thick line) HL-60 cells were incubated with anti-CD63 or anti-NE as primary antibody, then labeled with Alexa Fluor 488 as secondary antibody. When cells were stained on the surface only (nonpermeabilized; to the left), CD63 staining (upper panel) in CD63 siRNA cells decreased compared with wild-type and mock cells, but the levels in dom neg CD63–transfected cells were similar to wild-type and mock cells. The surface staining of NE (lower panel) was lower in both CD63 siRNA and CD63 dom neg cells than in wild-type and mock cells. After 8 weeks in culture, the clones reverted and regained their expression of CD63 and NE (although to a lesser degree), resembling mock and wild-type cells. Cells were also fixed and permeabilized to demonstrate the intracellular content of CD63 and NE (panels to the right). Regarding both CD63 and NE, the intracellular fluorescence of CD63 siRNA and CD63 dom neg cells was lower than that of wild-type and mock cells. Revertants recovered expression up to or equal to the same level as wild-type and mock cells. The plots are representative of 2 to 3 experiments.
NE of HL-60 cells is diminished by CD63 depletion—flow cytometry. Wild-type (wt), CD63 siRNA, CD63 siRNA mock (mock; thin line), reverted CD63 siRNA clone (siRNA rev; thick line), CD63 dom neg, CD63 dom neg mock (mock; thin line), and reverted CD63 dom neg (dom neg rev; thick line) HL-60 cells were incubated with anti-CD63 or anti-NE as primary antibody, then labeled with Alexa Fluor 488 as secondary antibody. When cells were stained on the surface only (nonpermeabilized; to the left), CD63 staining (upper panel) in CD63 siRNA cells decreased compared with wild-type and mock cells, but the levels in dom neg CD63–transfected cells were similar to wild-type and mock cells. The surface staining of NE (lower panel) was lower in both CD63 siRNA and CD63 dom neg cells than in wild-type and mock cells. After 8 weeks in culture, the clones reverted and regained their expression of CD63 and NE (although to a lesser degree), resembling mock and wild-type cells. Cells were also fixed and permeabilized to demonstrate the intracellular content of CD63 and NE (panels to the right). Regarding both CD63 and NE, the intracellular fluorescence of CD63 siRNA and CD63 dom neg cells was lower than that of wild-type and mock cells. Revertants recovered expression up to or equal to the same level as wild-type and mock cells. The plots are representative of 2 to 3 experiments.
Ultrastructural features of CD63-depleted HL-60 cells
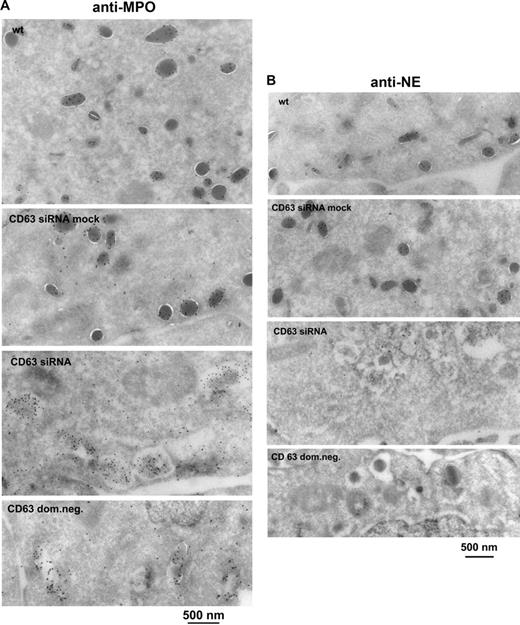
The phenotype of CD63-deficient HL-60 cells was examined by immunoelectron microscopy. The fields chosen in Figure 7A and B illustrate the ultrastructure of most of the different kinds of cells observed. Wild-type and CD63 siRNA mock cells contained electron-dense granules with reactivity for MPO (Figure 7A) and NE (Figure 7B) typical of secretory lysosomes. In contrast, CD63 siRNA and CD63 dom neg mutants generally lacked granules, and instead nearly all cells contained cytoplasmic vacuoles with a variable content of electron-dense aggregates with reactivity for MPO (Figure 7A) and only faint reactivity for NE (Figure 7B). The fraction of MPO-positive vacuoles and granules containing NE was determined by counting gold particles (Table 1). Of sections from 100 different cells of a CD63 siRNA clone, 73 contained NE-negative vacuoles and 27 contained NE-positive vacuoles. Five of the sections with NE-positive vacuoles contained a single NE-positive electron-dense granule and one section revealed a wild-type–like pattern with 29 NE-positive electron-dense granules. Similarly, scanning of sections from 100 different cells of a CD63 dom neg cell clone showed 80 with NE-negative vacuoles and 20 with NE-positive vacuoles. Three of the sections with NE-positive vacuoles also contained a single NE-positive electron-dense granule. Our results suggest that CD63 depletion distorted the biosynthesis of morphologically normal primary granules. The presence of cytoplasmic vacuoles may not, however, have been specific for CD63 depletion, and we occasionally observed such vacuoles in HL-60 wild-type clones. The lack of NE signal in 70% to 80% of the MPO-positive cytoplasmic vacuoles of CD63-depleted cells added weight to the hypothesis that the trafficking of NE to granules was being blocked. The results were consistent with those of the analyses showing a decrease of total cellular NE as well as reduced processing and constitutive secretion of proNE (Figure 5).
CD63-depleted HL-60 cells show a lack of morphologically normal granules and contain MPO-positive cytoplasmic vacuoles lacking NE—immunoelectron microscopy. Wild-type (wt), CD63 siRNA mock, CD63 siRNA, and CD63 dom neg HL-60 cells were examined. Ultrathin cryosections were labeled (A) with rabbit anti-MPO (10-nm gold) or (B) with rabbit anti-NE (10-nm gold). The CD63-depleted cells lacked normal granules and contained more vacuoles. MPO labeling was localized both to the granules of the wild-type and mock cells and to the vacuoles of CD63 siRNA and CD63 dom neg cells. NE labeling was seen in granules of wild-type and mock cells. Table 1 shows the NE-positive fraction of MPO-positive vacuoles/granules, obtained by counting gold particles in cell sections from 100 different cells of a CD63 siRNA clone and a CD63 dom neg clone. A lack of NE labeling was observed in 70% to 80% of the cytoplasmic vacuoles/granules of CD63 siRNA and CD63 dom neg cells. Labeling was sometimes observed on the Golgi (G) and trans-Golgi network (TGN) of CD63-depleted cells. Scrambled siRNA controls are shown in Figure S2F.
CD63-depleted HL-60 cells show a lack of morphologically normal granules and contain MPO-positive cytoplasmic vacuoles lacking NE—immunoelectron microscopy. Wild-type (wt), CD63 siRNA mock, CD63 siRNA, and CD63 dom neg HL-60 cells were examined. Ultrathin cryosections were labeled (A) with rabbit anti-MPO (10-nm gold) or (B) with rabbit anti-NE (10-nm gold). The CD63-depleted cells lacked normal granules and contained more vacuoles. MPO labeling was localized both to the granules of the wild-type and mock cells and to the vacuoles of CD63 siRNA and CD63 dom neg cells. NE labeling was seen in granules of wild-type and mock cells. Table 1 shows the NE-positive fraction of MPO-positive vacuoles/granules, obtained by counting gold particles in cell sections from 100 different cells of a CD63 siRNA clone and a CD63 dom neg clone. A lack of NE labeling was observed in 70% to 80% of the cytoplasmic vacuoles/granules of CD63 siRNA and CD63 dom neg cells. Labeling was sometimes observed on the Golgi (G) and trans-Golgi network (TGN) of CD63-depleted cells. Scrambled siRNA controls are shown in Figure S2F.
Discussion
In the present work, we identified a possible role for the tetraspanin CD63 in cellular retention and granule targeting of proNE of hematopoietic cells. First, coexpression in COS cells showed that there was an interaction between these 2 proteins and that proNE was retained in the cells by CD63. Second, depletion of CD63 in promyelocytic HL-60 cells by RNA interference or a dominant-negative CD63 mutant resulted in a decrease in mature NE. Third, depletion of CD63 resulted in both reduced processing and reduced constitutive secretion of proNE. Finally, the MPO-positive cytoplasmic organelles of CD63-depleted cells showed a strongly reduced NE signal, which is consistent with a block in trafficking. Therefore, our findings suggest that the targeting of proNE may occur via the protein-delivery pathway used by the integral membrane protein CD63. HL-60 cells are leukemic, but they can be induced to mature into neutrophils and monocytes,34 and studies of them are assumed to be relevant for the corresponding normal cells.
CD63 is lysosome targeted by the interaction of a C-terminal sequence, GYEVM, with the AP-3 adaptor complex.21 Tetraspanin complexes act as molecular facilitators in many cellular processes,35,36 and different partner proteins can become clustered together with CD63.37 It has been proposed that membrane proteins such as the H,K-ATPase25 and membrane type 1-matrix metalloproteinase23,38 are transported by CD63. Our study suggests that soluble proteins may also be escorted by CD63. The finding of an interaction between proNE and CD63 may add weight to this idea, as both proteins have a common destination in neutrophils,21 namely primary granules. Neutrophils contain the proteoglycan serglycin, which also has been proposed to have a role in the sorting and packing of cationic granule proteins.39,40 An association has been reported between NE and serglycin en route to lysosomes.32 New observations on serglycin knockout support the idea that this proteoglycan has a role in the targeting of NE to primary granules: mature neutrophils of knockout mice lacked NE.31 The precise mechanism by which the knockout phenotype is formed has not yet been revealed. Collectively, the information available suggests that the disruption of either AP-3,15,16 serglycin,31 or NE (by mutations in cyclic neutropenia)41 may perturb intracellular NE trafficking, suggesting intricate sorting. In addition, our findings also suggest that CD63 is involved in proNE trafficking. Therefore, the block in NE trafficking observed in AP-3 mutations15,16 might result from deficient cotargeting of proNE by CD63, which uses an AP-3–dependent delivery system.
Overexpression of CD63 increased the intracellular level of cotransfected NE in COS cells. CD63 depletion might therefore be expected to lead to the opposite, decreased retention and increased constitutive secretion. Instead, CD63 depletion in HL-60 cells was shown to block constitutive secretion. These seemingly contrasting results may be explained by limitations in the cell models. COS cells, unlike HL-60 cells, are not equipped for normal NE processing, but results obtained in these cells still suggest a role for CD63 in post-Golgi retention and targeting of proNE. Results obtained in HL-60 cells suggested that CD63 depletion induced a trafficking block. This block prohibited examination of post-Golgi retention in these cells. Thus, the results from COS cells may reflect a distal involvement of CD63 in proNE retention and granule targeting, whereas results from HL-60 cells may reflect a proximal involvement of CD63 in proNE trafficking at the level of the ER/Golgi. We identified structural requirements for the interaction between proNE and CD63 in COS cells. CD63 deficient of the large extracellular loop was still found in a complex with proNE but, unlike wild-type CD63, did not mediate cellular retention of proNE. It is possible that an interaction in this case was of lower affinity than that with wild-type CD63.
The steady-state level of proNE protein was importantly not reduced in CD63-depleted cells despite continuous degradation, which was however counteracted by the lack of constitutive secretion. Furthermore, the synthesis of proNE was unaffected. A speculative explanation for the proNE degradation in CD63-depleted cells might be that CD63 acts as a chaperone, normally protecting proNE from degradation during transport for storage. Moreover, the accumulation of proNE but not NE resulted from blocked secretory lysosome targeting with a lack of access to the processing enzymes assumed to be active in secretory lysosomes.4 An important question then arising was whether the CD63-depleted cells had a problem in creation of, or in transport to, the compartment of destination. Our study supports both possibilities. The fact that both granule targeting and constitutive secretion of proNE were affected in CD63-depleted HL-60 cells suggests that CD63 interaction takes place in the secretory route prior to the Golgi, in the ER, as routes for constitutive secretion and granule targeting may part in Golgi. Our findings also support the possibility that CD63 is required for establishing the compartment for NE storage. Thus, our data suggest a role for CD63 in the biogenesis of granules in as much as depletion of CD63 resulted in the replacement of morphologically normal granules with MPO-positive cytoplasmic vacuoles. As a result of a concomitant trafficking block in CD63-depleted cells, these vacuoles showed an expected lack of proNE.
Another question is why the sorting efficiency for luminal secretory lysosome proteins such as NE normally is low and results in major secretion of precursor forms by default.4 The increased capacity for proNE retention in COS cells coexpressing CD63 supported the possibility that the tetraspanin was involved. A gradual dissociation of a transient CD63/proNE complex during passage through intracellular compartments en route from the synthesis site in the ER to secretory lysosomes may explain the low sorting efficiency for proNE. As mature NE is stored as a soluble protein in the lumen of CD63+ secretory lysosomes/granules of neutrophils,4 dissociation from CD63 would be required for the release into the granule matrix and processing of proNE into mature enzyme. This release could result from conformational change during the proteolytic processing of proNE into mature NE or from granule acidification. However, we have not yet been able to prove a direct interaction between proNE and CD63.
We suggest that an interaction with the integral membrane tetraspanin CD63 is involved in the retention and sorting of proNE, which is stored as soluble mature enzyme in the primary granules of neutrophils. Similar retention and sorting mechanisms may be found for other soluble proteases of secretory lysosomes. Interestingly, the lysosomal integral membrane protein LIMP-2 was recently shown to be a specific partner of the soluble lysosomal enzyme β-glucocerebrosidase.42
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Swedish Cancer Foundation, Stockholm, Sweden; the Swedish Childhood Cancer Foundation, Stockholm, Sweden; the Swedish Research Council (grants nos. 1329 and 12613), Stockholm, Sweden; the Alfred Österlund Foundation, Lund, Sweden; the Gustav V Fund, Stockholm, Sweden; The Royal Swedish Academy of Sciences, Stockholm, Sweden; and funds from Lund University Hospital.
Authorship
Contribution: L.K., M.H., H.T., and I.O. designed research; L.K., A.-M.P., H.J., and J.C. performed research; L.K., M.H., H.T., and I.O. analyzed data; L.K., M.H., H.T., and I.O. wrote the paper; and all authors checked the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus Hansson, Department of Hematology, BMC C14, SE-221 84 Lund, Sweden; e-mail: markus.hansson@med.lu.se.

![Figure 1. CD63 and proNE form a complex. (A) A schematic diagram of the constructs used. The NE (preproNE) construct is shown with signal peptide, N-terminal dipropeptide (SE), mature enzyme sequence, and C-terminal propeptide. The full-length CD63 and the CD63 mutants; CD63ΔLEL, lacking the large extracellular loop (LEL), CD63Δ12 with deleted C-terminal lysosomal targeting motif, and CD63 dom neg (dominant-negative inhibition) with a single amino acid change in the C-terminal sorting motif are shown. N-terminal fusions of the CD63 variants with GFP were used for transfection of COS cells. The CD63 siRNA construct used is also shown with the complementary mRNA region forming a hairpin and marking the corresponding mRNA target region in the protein. (B) COS cells were transfected with plasmids carrying GFP-CD63 (CD63), NE, or GFP-CD63ΔLEL, as indicated. Cell lysates were examined by immunoblotting with anti-actin, anti-GFP (for detection of GFP-CD63 fusion proteins), and anti-cproNE, showing comparable expression of the plasmids. IP/Western was performed to examine coprecipitation between CD63 and proNE. Both GFP-CD63 and GFP-CD63ΔLEL coprecipitated proNE, as judged by IP with anti-GFP followed by Western blotting with anti-cproNE. In addition, proNE coprecipitated the GFP-CD63 and GFP-CD63ΔLEL, as judged by IP with anti-cproNE followed by immunoblotting with anti-GFP. (C) COS cells transfected as indicated were incubated for 24 hours with [35S]methionine/[35S]cysteine to achieve steady-state radiolabeling of proteins and then examined by IP and fluorography. A major radiolabeled 31-kDa proNE band was detected in cells expressing proNE or proNE plus GFP-CD63 (marked to the right with proNE). Upon coexpression, IP with anti-cproNE copurified proteins migrating as GFP-CD63 (●) or GFP-CD63ΔLEL (▲), and IP with anti-GFP copurified proteins migrating as proNE. In addition, GFP-CD63ΔLEL (▲) copurified proNE and vice versa. These data confirm complex formation between proNE and GFP-CD63 or GFP-CD63ΔLEL. The 85-kDa band of the supernatant corresponds to a covalent complex between proNE and α1-proteinase inhibitor.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/8/10.1182_blood-2007-10-116285/4/m_zh80200825580001.jpeg?Expires=1765980242&Signature=1RDFh6dwnzn9iSew9GgiR2s2AvSKKUpAqEWS1AquNAZnIu983O22xor~CNDeR-ofgUS-x-5GAkWSwyPbAcmJeK91fIgV6FYGBwGAvCkYnAvXygyhzf-KGuqq3LEHwt3IOmZ17yCAptj6JDYwRbDW9eDYAiF~VyYaiwe9wMNc3ReSEV5QzVp2AP9v3V1Hazn19NrxQvBkMxBLeEJJvjFIeuIbGiI8383rurafo9MRCgWK2ZvOQ0iKX7Ab0CjsGYn7LPmN0Vtp9wycBnqMn6tXFzBlr5spdEPucwlemZ1AOiG2XZ1eK39-eF9Zn-~i2LtptJhQmN5XgqWLaue8nzhAYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)