Abstract
The chimeric monoclonal antibody rituximab is the standard of care for patients with B-cell non-Hodgkin lymphoma (B-NHL). Rituximab mediates complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity of CD20-positive human B cells. In addition, rituximab sensitizes B-NHL cells to cytotoxic chemotherapy and has direct apoptotic and antiproliferative effects. Whereas expression of the CD20 antigen is a natural prerequisite for rituximab sensitivity, cell-autonomous factors determining the response of B-NHL to rituximab are less defined. To this end, we have studied rituximab-induced apoptosis in human B-NHL models. We find that rituximab directly triggers apoptosis via the mitochondrial pathway of caspase activation. Expression of antiapoptotic Bcl-xL confers resistance against rituximab-induced apoptosis in vitro and rituximab treatment of xenografted B-NHL in vivo. B-NHL cells insensitive to rituximab-induced apoptosis exhibit increased endogenous expression of multiple antiapoptotic Bcl-2 family proteins, or activation of phosphatidylinositol-3-kinase signaling resulting in up-regulation of Mcl-1. The former resistance pattern is overcome by treatment with the BH3-mimetic ABT-737, the latter by combining rituximab with pharmacologic phosphatidylinositol-3-kinase inhibitors. In conclusion, sensitivity of B-NHL cells to rituximab-induced apoptosis is determined at the level of mitochondria. Pharmacologic modulation of Bcl-2 family proteins or their upstream regulators is a promising strategy to overcome rituximab resistance.
Introduction
Treatment options for patients with CD20-positive malignant lymphoma have greatly benefited from the clinical introduction of the chimeric monoclonal antibody rituximab.1 Rituximab has significant single-agent activity in several indolent lymphoma entities2-5 but is less active in aggressive lymphoma.6 When combined with cytotoxic chemotherapy, rituximab dramatically improves the outcome of patients with indolent and aggressive B-cell non-Hodgkin lymphoma (B-NHL). This is true for first line therapies based on alkylating agents, vincristine and anthracyclines, as well as for salvage regimens, including high-dose therapy with hematopoietic stem cell support for patients with relapsing lymphoma.7-12 Typically, lymphoma patients respond to several lines of rituximab-based therapies. However, resistance to rituximab eventually evolves during the course of disease, which is only in part explained by the loss of CD20 expression.13 Because of the more widespread use of rituximab maintenance therapy for indolent lymphomas, increased selection for antibody resistance can be expected. In addition, patients with CD20-positive B-NHL with adverse prognostic features still exhibit dismal outcomes despite rituximab-based first-line therapies.
Current understanding attributes the clinical efficacy of rituximab to indirect as well as direct effector mechanisms. Rituximab mediates complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) of CD20-positive B cells. These indirect activities rely on the human constant fragment (Fc) of rituximab, which binds the complement factor C1q as well as Fc receptors on macrophages and natural killer (NK) cells.14 Direct activities of rituximab are less understood. Possibly, rituximab triggers intracellular signaling events by recruitment and clustering of the CD20 antigen in lipid rafts. Several in vitro models have demonstrated that rituximab may either directly induce apoptosis or sensitize B-NHL cells to apoptosis induced by cytotoxic anticancer agents.15-18 Mechanistically, rituximab was shown to abrogate intracellular signal transduction of survival pathways impinging on NF-κB, mitogen-activated protein kinases (MAPK), and protein kinase B/Akt (Akt).19 The importance of such direct effector mechanisms is underscored by the clinical observation of rituximab failure despite maintained CD20 antigen expression.13,20
Against this background, we set out to identify endogenous resistance mechanisms, which determine the response of B-NHL cells to rituximab treatment. The knowledge of such mechanisms will guide identification of molecular targets for therapeutic interventions overcoming primary or secondary antibody resistance. We found that rituximab directly triggers the “mitochondrial” pathway of apoptotic caspase activation in B-NHL cells. Accordingly, functional defects in apoptotic signal transduction mediated resistance to rituximab therapy in vitro and in vivo. Molecular dissection of rituximab-resistant B-NHL cells identified the simultaneous expression of multiple antiapoptotic Bcl-2 family proteins, as well as up-regulation of Mcl-1 by deregulated phosphatidylinositol-3-kinase (PI3K) signaling as independent resistance mechanisms, which were successfully reversed by molecularly targeted pharmacotherapies.
Methods
Cell lines
The human B-NHL cell lines SU-DHL-4 (diffuse large B-cell lymphoma (DLBCL)), Ramos (Burkitt lymphoma), WSU-NHL (follicular lymphoma [FL]), Jeko-1 (mantle cell lymphoma), HT (DLBCL), and Sc-1 (FL) were obtained from the DSMZ (Braunschweig, Germany) and maintained in RPMI 1640 media (Invitrogen, Karlsruhe, Germany) supplemented with fetal bovine serum (Invitrogen), penicillin/streptomycin, and l-glutamine (Invitrogen). The packaging cell line FNX ampho (provided by Dr G. P. Nolan, Stanford University, Stanford, CA) was grown in fully supplemented Dulbecco modified Eagle medium (Invitrogen). Replication-defective retroviral particles were generated by transient transfection as described previously.21 B-NHL cells were incubated with retrovirus-containing supernatants, and successfully transduced, enhanced green-fluorescent protein (EGFP)-positive cell populations were obtained by fluorescence-activated cell sorting (FACSAria, BD Biosciences, San Jose, CA).
Antibodies, vectors, and reagents
Rituximab (MabThera; Roche Diagnostics, Mannheim, Germany; stock solution 10 mg/mL) was purchased from the Hospital Pharmacy of the Johannes Gutenberg University; a goat antihuman Ig F(ab′)2 fragment (Invitrogen) was used for crosslinking. An anti-epidermal growth factor receptor monoclonal IgG1 antibody (Cetuximab; generously provided by Merck, Darmstadt, Germany) was used as isotype control. The following primary antibodies were applied for immunoblotting or immunohistochemistry: actin (C4, ICN); Akt1/2 (H-136), Bcl-2 (C2), Mcl-1 (all from Santa Cruz Biotechnology, Santa Cruz, CA); Bcl-xL (54H6), Bfl-1, PTEN, GSK3-β (all from Cell Signaling Technology, Danvers, MA); Bak (Ab-1; Calbiochem, San Diego, CA), Bax (Upstate Biotechnology, Charlottesville, VA), CD20 (L26; Dako North America, Carpinteria, CA); Ki-67 (BGX-297; Biogenex, San Ramon, CA); all phosphoepitope-specific antisera were purchased from Cell Signaling Technology. Bicistronic retroviral vectors expressing antiapoptotic Bcl-xL, DN-FADD, and DN-caspase-9 have been previously published.22 ABT-737 was generously provided by Abbott Laboratories (Abbott Park, IL); wortmannin, LY294,002, and staurosporine were purchased from Calbiochem, Cayman Chemicals (Ann Arbor, MI), and Sigma-Aldrich (St Louis, MO), respectively.
Cytotoxicity and apoptosis assays
For CDC and ADCC assays, B-NHL cell lines were incubated with rituximab (10 μg/mL), control antibody, and human serum (5%) or isolated mononuclear cells from normal donor buffy coats (effector-to-target ratio = 10:1), respectively. Cell death was quantified flow cytometrically after staining with propidium iodide (PI; Sigma-Aldrich). Apoptosis was measured by a fluorescently labeled caspase substrate (FITC-VAD; Oncogene Science, Cambridge, MA), and by detection of fragmented DNA after hypotonic lysis and staining with PI. Cells with maintained mitochondrial transmembrane potential Δψm were quantified flow cytometrically using the mitochondrial marker tetramethylrhodamine ethylester (TMRE; Invitrogen) as described previously.22 For preparation of cellular extracts, pellets were resuspended in chilled cell extract buffer (20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-KOH, pH 7.5, 10 mM KCl, 1 mM of MgCl2, 1 mM ethyleneglycoltetraacetic acid, 0.25 M sucrose, 2 mM dithiothreitol, 10 μM cytochalasin B, complete protease inhibitors; Roche Diagnostics) and vigorously broken using a Dounce homogenizer. For caspase activation, extracts (protein concentration ≥ 10 mg/mL) were incubated with 10 μM cytochrome c and 1 mM dATP (Sigma-Aldrich) at 37°C. Caspase-3-like activity was measured kinetically by a colorimetric assay using DEVD-pNA (Calbiochem) as substrate.23 Cytosolic cytochrome c was determined in supernatants from selectively permeabilized cells using an enzyme-linked immunosorbent assay (Bender MedSystems, Vienna, Austria) following the manufacturer's instructions.
Murine lymphoma model
Irradiated (150 cGy) NOD/SCID mice were intravenously inoculated with 107 Ramos or HT B-NHL cells and monitored daily for clinical symptoms. In the treatment protocol, lymphoma-bearing mice received intraperitoneal injections of rituximab (20 mg/kg body weight), isotype controle, or vehicle. Injections were administered once daily from day 5 to day 10, followed by twice weekly injections from day 14 on. For cotreatment studies, mice received simultaneous intraperitoneal injections of LY294,002 (20 mg/kg body weight) or vehicle. Mice were killed as soon as they exhibited symptoms of disseminated lymphoma growth. Organ samples from lymphoma-bearing and control mice were histopathologically and immunohistochemically examined following diagnostic protocols of the Department of Pathology, University Hospital Essen. All animal studies were conducted in compliance with institutional guidelines and were approved by the regulatory authority (Landesuntersuchungsamt Rheinland-Pfalz, Az. 1.5 177-07/051-54). Kaplan-Meier plots were analyzed using the log rank test (Mantel Cox).
Analysis of clinical samples
Immunohistochemical analyses of expression of Bcl-2, Bcl-xL, Mcl-1, PTEN, Akt, and p-AktS473 were carried out in tumor biopsies following standard protocols of the Department of Pathology, University Hospital Essen. Tumor-specific expression of each protein was quantified using the immunoreactive score.24 Clinical samples comprised surplus biopsy material from lymphoma patients treated in clinical protocols of rituximab-based salvage therapy for relapsing B-NHL.11 The studies had been approved by the responsible regulatory body (Ethikkommission der Landesärztekammer Rheinland-Pfalz), and all patients had provided written informed consent in accordance with the Declaration of Helsinki.
Results
Rituximab is a direct inducer of apoptosis in vitro and in vivo
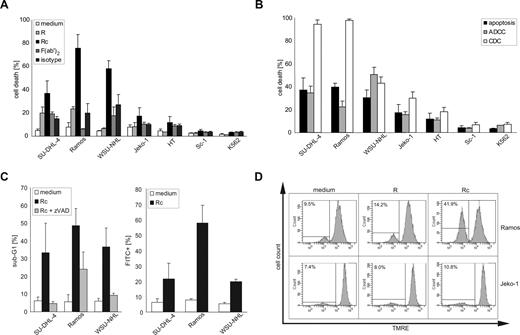
The clinical activity of rituximab is in part attributed to direct effects on CD20-positive B-NHL cells. Treating 6 established B-NHL cell lines (SU-DHL-4, Ramos, WSU-NHL, Jeko-1, Sc-1, HT) with rituximab under various conditions, we observed significant cell death in 3 cell lines (SU-DHL-4, Ramos, WSU-NHL) only when the antibody was cross-linked using an anti–human immunoglobulin F(ab′)2 fragment. The remaining cell lines (Jeko-1, Sc-1, HT) were largely insensitive to rituximab (Figure 1A). Direct induction of cell death was rituximab-specific, as the secondary antibody alone or a cross-linked isotype control antibody exhibited negligible cytotoxicity, and the CD20-negative leukemia cell line K562 was completely protected (Figure 1A). Because CDC and ADCC are considered major indirect effector pathways triggered by the clinical application of rituximab, we studied whether resistance to direct induction of apoptosis by rituximab correlated with such indirect mechanisms of action. Whereas CDC proved to be very effective in some rituximab-sensitive B-NHL cell lines (SU-DHL-4, Ramos), rituximab-mediated ADCC exhibited no superior activity compared with cross-linked rituximab in the present experimental system (Figure 1B). Importantly, B-NHL cell lines resistant to direct induction of apoptosis by rituximab also proved less sensitive to specific rituximab-mediated CDC and ADCC (Figure 1B; and Figure S1A,B, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Direct and indirect induction of cell death by rituximab in B-NHL cell lines. (A) Human B-NHL cell lines were incubated with monomeric rituximab (R), rituximab cross-linked by an anti-Ig F(ab′)2 fragment (Rc), the anti-Ig F(ab′)2 fragment alone (F(ab′)2), or the isotype control for 48 hours. The CD20-negative human leukemia cell line K562 served as negative control. Cell death was quantified flow cytometrically after staining with PI; mean values plus SD of 3 independent experiments are shown. (B) Human B-NHL cell lines were incubated with cross-linked rituximab (apoptosis), monomeric rituximab, and mononuclear cells (ADCC), or monomeric rituximab and human serum (CDC). The CD20-negative human leukemia cell line K562 served as negative control. Cell death was quantified flow cytometrically after staining with PI; mean values plus SD of 3 independent experiments are shown. (C) The 3 rituximab-sensitive B-NHL cell lines (SU-DHL-4, Ramos, WSU-NHL) were incubated with cross-linked rituximab in the presence of vehicle (Rc) or the broad-spectrum caspase inhibitor zVAD-fmk (50 μM, Rc + zVAD) for 48 hours. Cells with apoptotic DNA fragmentation were quantified flow cytometrically after hypotonic lysis and staining with PI. Mean values plus SD of 3 independent experiments are given (left panel). The same B-NHL cell lines were incubated with cross-linked rituximab (Rc) for 24 hours, and the fraction of cells with caspase-3-like activity was determined flow cytometrically after staining with the fluorescent caspase substrate FITC-VAD. Mean values plus SD of 3 independent experiments are shown (right panel). (D) Rituximab-sensitive Ramos B-NHL cells and rituximab-resistant Jeko-1 B-NHL cells were incubated with monomeric (R) or cross-linked rituximab (Rc) for 24 hours. The fraction of cells with dissipated mitochondrial transmembrane potential Δψm was determined flow cytometrically by loss of staining with the fluorescent mitochondrial dye TMRE. Representative histograms of at least 3 independent repeat experiments are shown. Note the loss of TMRE staining in Ramos, but not in Jeko-1 cells after treatment with cross-linked rituximab.
Direct and indirect induction of cell death by rituximab in B-NHL cell lines. (A) Human B-NHL cell lines were incubated with monomeric rituximab (R), rituximab cross-linked by an anti-Ig F(ab′)2 fragment (Rc), the anti-Ig F(ab′)2 fragment alone (F(ab′)2), or the isotype control for 48 hours. The CD20-negative human leukemia cell line K562 served as negative control. Cell death was quantified flow cytometrically after staining with PI; mean values plus SD of 3 independent experiments are shown. (B) Human B-NHL cell lines were incubated with cross-linked rituximab (apoptosis), monomeric rituximab, and mononuclear cells (ADCC), or monomeric rituximab and human serum (CDC). The CD20-negative human leukemia cell line K562 served as negative control. Cell death was quantified flow cytometrically after staining with PI; mean values plus SD of 3 independent experiments are shown. (C) The 3 rituximab-sensitive B-NHL cell lines (SU-DHL-4, Ramos, WSU-NHL) were incubated with cross-linked rituximab in the presence of vehicle (Rc) or the broad-spectrum caspase inhibitor zVAD-fmk (50 μM, Rc + zVAD) for 48 hours. Cells with apoptotic DNA fragmentation were quantified flow cytometrically after hypotonic lysis and staining with PI. Mean values plus SD of 3 independent experiments are given (left panel). The same B-NHL cell lines were incubated with cross-linked rituximab (Rc) for 24 hours, and the fraction of cells with caspase-3-like activity was determined flow cytometrically after staining with the fluorescent caspase substrate FITC-VAD. Mean values plus SD of 3 independent experiments are shown (right panel). (D) Rituximab-sensitive Ramos B-NHL cells and rituximab-resistant Jeko-1 B-NHL cells were incubated with monomeric (R) or cross-linked rituximab (Rc) for 24 hours. The fraction of cells with dissipated mitochondrial transmembrane potential Δψm was determined flow cytometrically by loss of staining with the fluorescent mitochondrial dye TMRE. Representative histograms of at least 3 independent repeat experiments are shown. Note the loss of TMRE staining in Ramos, but not in Jeko-1 cells after treatment with cross-linked rituximab.
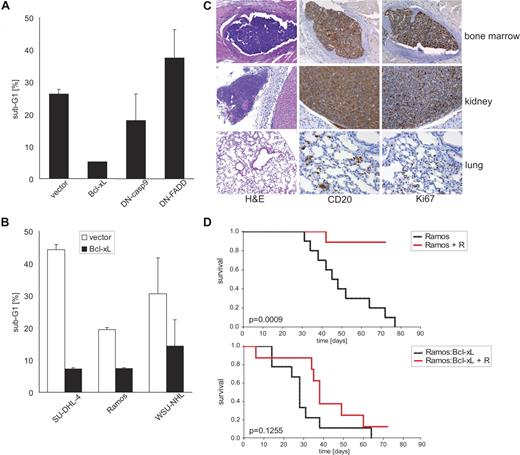
To further characterize rituximab-induced cell death in this model, we studied common features of apoptosis, such as DNA fragmentation, caspase activation, and loss of the mitochondrial transmembrane potential Δψm. Rituximab treatment triggered internucleosomal DNA fragmentation (Figure 1C), dissipation of Δψm (Figure 1D), and caspase-3–like activity (Figure 1C) in SU-DHL-4, Ramos, and WSU-NHL B-NHL cells. Moreover, rituximab-induced cell death was effectively prevented by pretreatment with the caspase inhibitor zVAD-fmk (Figure 1C). To dissect the molecular pathway of rituximab-induced apoptosis, we devised retroviral gene transfer to express genetic inhibitors of apoptosis in antibody-sensitive B-NHL cells. Antiapoptotic Bcl-xL significantly protected SU-DHL-4, Ramos, and WSU-NHL cells against rituximab cytotoxicity (Figure 2A,B). In contrast, the expression of a dominant-negative mutant Fas-associated Death Domain (DN-FADD) had no such effect (Figure 2A), whereas a dominant-negative mutant caspase-9 (DN-caspase-9) conferred some protection. Collectively, these findings suggested that cross-linked rituximab is a direct inducer of apoptosis of B-NHL cells, and its activity can be counteracted by inhibitors of the mitochondrial pathway of caspase activation, such as Bcl-xL. Because transgenic expression of Bcl-xL had no effect on endogenous CD20 expression levels of B-NHL cells (Figure S1C), protection was most likely conferred by its antiapoptotic activity.
Antiapoptotic Bcl-xL protects B-NHL cells to rituximab-induced apoptosis in vitro and in vivo. (A) Rituximab-sensitive SU-DHL-4 cells were retrovirally transduced to express the indicated genetic inhibitors of apoptosis and EGFP via a bicistronic cassette. EGFP-expressing cells were isolated by fluorescence-activated cell sorting followed by treatment with cross-linked rituximab for 48 hours. The fraction of cells with apoptotic DNA fragmentation was quantified flow cytometrically after hypotonic lysis and staining with PI; mean values plus SD of 3 independent experiments are given. (B) Three rituximab-sensitive B-NHL cell lines were retrovirally transduced to express antiapoptotic Bcl-xL and EGFP or control vector, and were sorted as in panel A. The fraction of cells with apoptotic DNA fragmentation was quantified flow cytometrically after treatment with cross-linked rituximab for 48 hours; mean values plus SD of 3 independent experiments are shown. (C) Histopathologic analysis of NOD/SCID mice that were intravenously inoculated with human Ramos B-NHL cells. Representative photomicrographs from organ sections stained with hematoxylin/eosin and antibodies against the human CD20 antigen or the mitotic marker Ki-67. Note the presence of mitotically active human CD20-positive B-NHL cells demonstrating engraftment in various murine organs. Slides were viewed with a Zeiss Axioplan upright research-grade microscope for brightfield and fluorescence applications (Carl Zeiss, Jena, Germany) using a Plan-NEOFLUAR lens at 10 ×/0.30, 20 ×/0.50, and 40 ×/0.75. Images were acquired using the Axio Cam HRc camera (Zeiss), and were processed with Axio-Vision Rel. 4.6 software (Zeiss). (D) Kaplan-Meier plots of overall survival of NOD/SCID mice after intravenous inoculation of 107 Ramos (top panel) or Bcl-xL–expressing Ramos cells (bottom panel). Starting on day 5 after tumor inoculation, the mice received intraperitoneal injections of rituximab (+ R, red line) or vehicle (black line); 10 mice per group. Note that rituximab treatment significantly (P = .001) prolonged survival of Ramos lymphoma-bearing, but not of Ramos-Bcl-xL lymphoma-bearing mice.
Antiapoptotic Bcl-xL protects B-NHL cells to rituximab-induced apoptosis in vitro and in vivo. (A) Rituximab-sensitive SU-DHL-4 cells were retrovirally transduced to express the indicated genetic inhibitors of apoptosis and EGFP via a bicistronic cassette. EGFP-expressing cells were isolated by fluorescence-activated cell sorting followed by treatment with cross-linked rituximab for 48 hours. The fraction of cells with apoptotic DNA fragmentation was quantified flow cytometrically after hypotonic lysis and staining with PI; mean values plus SD of 3 independent experiments are given. (B) Three rituximab-sensitive B-NHL cell lines were retrovirally transduced to express antiapoptotic Bcl-xL and EGFP or control vector, and were sorted as in panel A. The fraction of cells with apoptotic DNA fragmentation was quantified flow cytometrically after treatment with cross-linked rituximab for 48 hours; mean values plus SD of 3 independent experiments are shown. (C) Histopathologic analysis of NOD/SCID mice that were intravenously inoculated with human Ramos B-NHL cells. Representative photomicrographs from organ sections stained with hematoxylin/eosin and antibodies against the human CD20 antigen or the mitotic marker Ki-67. Note the presence of mitotically active human CD20-positive B-NHL cells demonstrating engraftment in various murine organs. Slides were viewed with a Zeiss Axioplan upright research-grade microscope for brightfield and fluorescence applications (Carl Zeiss, Jena, Germany) using a Plan-NEOFLUAR lens at 10 ×/0.30, 20 ×/0.50, and 40 ×/0.75. Images were acquired using the Axio Cam HRc camera (Zeiss), and were processed with Axio-Vision Rel. 4.6 software (Zeiss). (D) Kaplan-Meier plots of overall survival of NOD/SCID mice after intravenous inoculation of 107 Ramos (top panel) or Bcl-xL–expressing Ramos cells (bottom panel). Starting on day 5 after tumor inoculation, the mice received intraperitoneal injections of rituximab (+ R, red line) or vehicle (black line); 10 mice per group. Note that rituximab treatment significantly (P = .001) prolonged survival of Ramos lymphoma-bearing, but not of Ramos-Bcl-xL lymphoma-bearing mice.
To assess whether this apoptotic pathway was also effective in vivo, we studied lymphoma xenografts established in irradiated NOD/SCID mice. These mice are devoid of endogenous B and T lymphocytes and have impaired NK-cell function. Moreover, complement activity is virtually absent in NOD/SCID mice,25 and residual immune cells were depleted by γ-radiation. Thus, any therapeutic activity of rituximab observed in our NOD/SCID model most probably resulted from direct antibody effects, which differed from other xenograft studies performed in SCID mice.26 After intravenous inoculation, engraftment and growth of CD20-positive human Ramos B-NHL cells in irradiated NOD/SCID mice were histopathologically confirmed in various organs (Figure 2C). After a latency period of 30 to 70 days, xenografted mice developed hind leg paralysis or additional symptoms of disseminated lymphoma spread and had to be killed (Figure 2D, top panel). Repetitive intraperitoneal injections of rituximab significantly (P = .001, log rank test) prolonged symptom-free survival of lymphoma-bearing NOD/SCID mice (Figure 2D, top panel). This effect was rituximab-specific as treatment with an isotype control antibody failed to protect xenografted mice (Figure S2). As anticipated from genetic murine B-cell lymphoma models,27 the expression of antiapoptotic Bcl-xL accelerated the onset of clinical symptoms from Ramos lymphoma in vivo (Figure 2D, bottom panel). Interestingly, rituximab treatment clearly was inactive in mice bearing Ramos-Bcl-xL lymphoma and failed to significantly prolong symptom-free survival (Figure 2D, bottom panel). Taken together, these findings support the relevance of direct antibody effects for the efficacy of rituximab treatment in vivo. Moreover, at least some of the antibody's antilymphoma activity in vivo appears to be mediated by Bcl-xL–inhibitable apoptosis.
Sensitivity to rituximab-induced apoptosis is determined at the level of mitochondria
Three of the 6 B-NHL cell lines (Jeko-1, Sc-1, HT) analyzed in this study exhibited primary resistance against rituximab-induced apoptosis (Figure 1A). Accordingly, we set out to characterize the intrinsic resistance mechanisms used by these B-NHL cells. Surface expression of the CD20 antigen was confirmed in all 6 B-NHL cell lines, thus ruling out loss of CD20 expression as explanation for the relative inactivity of the antibody (Table 1).
Intensity of CD20 expression in human B-NHL cell lines and the CD20-negative leukemia cell line K562
| Cell line . | MFI, arbitrary units . |
|---|---|
| SU-DHL-4 | 398 |
| Ramos | 271 |
| WSU-NHL | 185 |
| Jeko-1 | 195 |
| HT | 195 |
| Sc-1 | 12 |
| K562 | 0 |
| Cell line . | MFI, arbitrary units . |
|---|---|
| SU-DHL-4 | 398 |
| Ramos | 271 |
| WSU-NHL | 185 |
| Jeko-1 | 195 |
| HT | 195 |
| Sc-1 | 12 |
| K562 | 0 |
MFI indicates mean CD20-specific fluorescence intensity as determined by direct immunofluorescence and flow cytometry.
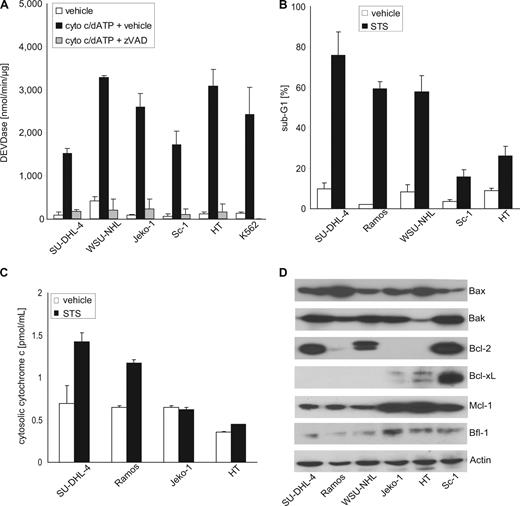
Effector caspases, such as caspase-3 or -7, are the essential executors of apoptotic cell death.28,29 To assess whether defects in caspase activity impacted on sensitivity to rituximab-induced apoptosis, we studied cytochrome c-dependent caspase activation in cytosolic extracts prepared from resistant and sensitive B-NHL cell lines. This assay recapitulates apoptosome-mediated activation of caspase-9 and subsequent effector caspases, and thus is a read-out for effector caspase activity in general, as well as for postmitochondrial caspase activation via the intrinsic pathway.30 Interestingly, extracts obtained from rituximab-sensitive and rituximab-resistant B-NHL cell lines were equally capable of activating caspase-3-like activity in response to exogenously added cytochrome c and dATP (Figure 3A). This observation excluded a role for insufficient effector caspase activity in rituximab-resistance of B-NHL cells in this study.
Sensitivity to rituximab-induced apoptosis is determined at the level of mitochondria. (A) Cell-free activation of caspase-3-like activity (DEVDase) by cytochrome c and dATP (cyto c/dATP) in extracts prepared from rituximab-sensitive and rituximab-resistant B-NHL cell lines is prevented by the caspase inhibitor zVAD-fmk (zVAD). The leukemia cell line K562 served as control (mean values + SD of triplicate experiments). (B) Induction of apoptosis in rituximab-sensitive (SU-DHL-4, Ramos, WSU-NHL) and rituximab-resistant B-NHL cells lines (Sc-1, HT) after incubation with the kinase inhibitor staurosporine (STS, 50 nM) for 48 hours. The fraction of cells with apoptotic DNA fragmentation was quantified flow cytometrically; mean values plus SD of 3 independent experiments are given. (C) Staurosporine (STS) induces the release of cytochrome c from the mitochondria into the cytoplasm in rituximab-sensitive SU-DHL-4 and Ramos B-NHL cells, but not in rituximab-resistant Jeko-1 and HT B-NHL cells (mean values + SD of 3 independent experiments). (D) Immunoblot analyses of the constitutive protein expression of proapoptotic Bax and Bak, and antiapoptotic Bcl-2, Bcl-xL, Mcl-1, and Bfl-1 in the human B-NHL cell lines. Actin served as loading control.
Sensitivity to rituximab-induced apoptosis is determined at the level of mitochondria. (A) Cell-free activation of caspase-3-like activity (DEVDase) by cytochrome c and dATP (cyto c/dATP) in extracts prepared from rituximab-sensitive and rituximab-resistant B-NHL cell lines is prevented by the caspase inhibitor zVAD-fmk (zVAD). The leukemia cell line K562 served as control (mean values + SD of triplicate experiments). (B) Induction of apoptosis in rituximab-sensitive (SU-DHL-4, Ramos, WSU-NHL) and rituximab-resistant B-NHL cells lines (Sc-1, HT) after incubation with the kinase inhibitor staurosporine (STS, 50 nM) for 48 hours. The fraction of cells with apoptotic DNA fragmentation was quantified flow cytometrically; mean values plus SD of 3 independent experiments are given. (C) Staurosporine (STS) induces the release of cytochrome c from the mitochondria into the cytoplasm in rituximab-sensitive SU-DHL-4 and Ramos B-NHL cells, but not in rituximab-resistant Jeko-1 and HT B-NHL cells (mean values + SD of 3 independent experiments). (D) Immunoblot analyses of the constitutive protein expression of proapoptotic Bax and Bak, and antiapoptotic Bcl-2, Bcl-xL, Mcl-1, and Bfl-1 in the human B-NHL cell lines. Actin served as loading control.
Rituximab-induced apoptosis of SU-DHL-4, Ramos, and WSU-NHL cells was accompanied by loss of Δψm (Figure 1D). Moreover, Bcl-xL, which prevents permeabilization of the mitochondrial outer membrane (MOM), effectively protected B-NHL cells against rituximab-induced apoptosis in vitro and in vivo (Figure 2A,B,D). These observations suggested that rituximab triggered caspase activation via the intrinsic, “mitochondrial” pathway.31 Common activators of this pathway are developmental stresses, growth factor withdrawal, DNA damage, or treatment with the broad-spectrum kinase inhibitor staurosporine. Studying staurosporine-induced apoptosis in B-NHL cells, we observed a pattern of sensitivity and resistance similar to the one revealed by rituximab treatment (Figure 3B). Moreover, apoptosis sensitivity correlated with the ability to release cytochrome c from the mitochondria into the cytosol (Figure 3C). Thus, the intrinsic sensitivity of B-NHL cells to apoptosis induced by rituximab or staurosporine appeared to be determined upstream of apoptosome-dependent caspase activation at the level of MOM permeabilization.
Modulation of rituximab resistance by pharmacologic targeting of Bcl-2 proteins
Permeabilization of the MOM is regulated by the pro- and antiapoptotic members of the Bcl-2 family.32 Analyzing endogenous expression of the essential proapoptotic BH1-2-3 proteins Bax and Bak,33 and of antiapoptotic Bcl-2 proteins relevant to the hematopoietic system, such as Bcl-2, Bcl-xL, Mcl-1, and Bfl-1 in rituximab-sensitive and resistant B-NHL cell lines, a diverse pattern emerged (Figure 3D): 2 rituximab-sensitive (SU-DHL-4, WSU-NHL) and one resistant (Sc-1) B-NHL cell lines harbored BCL-2 gene rearrangements and thus expressed high protein levels of Bcl-2. Interestingly, rituximab-resistant Sc-1 was the only cell line to express high protein levels of antiapoptotic Bcl-xL. Jeko-1 and HT cells, which were also insensitive to rituximab despite the absence of detectable Bcl-2 or Bcl-xL protein expression, exhibited the highest protein levels of antiapoptotic Mcl-1. Only low endogenous expression of antiapoptotic Bfl-1 was detected, and levels appeared somewhat higher in rituximab-resistant cell lines (Figure 3D). Thus, those B-NHL cell lines with endogenous resistance to rituximab-induced apoptosis either highly expressed 2 antiapoptotic Bcl-2 family proteins (Sc-1: Bcl-2 plus Bcl-xL), or high levels of Mcl-1 (Jeko-1, HT). In contrast, sensitive B-NHL cell lines (SU-DHL-4, Ramos, WSU-NHL) exhibited low levels of Mcl-1 and no detectable Bcl-xL expression (Figure 3D). Despite being described to correlate with acquired resistance after prolonged exposure to rituximab,34 the expression pattern of proapoptotic Bax and Bak failed to correlate with primary rituximab sensitivity and resistance in this study (Figure 3D).
To assess whether the combined expression of Bcl-2 and Bcl-xL determined resistance of Sc-1 B-NHL cells to rituximab-induced apoptosis, we made use of the pharmacologic BH3-mimetic ABT-737.35 This compound is a functional inactivator of Bcl-2, Bcl-xL, or Bcl-w, but not Mcl-1 or Bfl-1.36-38 Indeed, ABT-737 at low nanomolar concentrations effectively sensitized Sc-1 cells to apoptosis induced by rituximab (Figure 4A) or staurosporine (Figure 4B). In contrast, even 20-fold higher concentrations of ABT-737 failed to sensitize Jeko-1 and HT cells, which expressed high levels of Mcl-1 (Figures 3D, 4). Hence, the expression pattern of antiapoptotic Bcl-2 family members appears to dictate the sensitivity of B-NHL cells to rituximab-induced apoptosis. The BH3-mimetic ABT-737 can sensitize rituximab-resistant B-NHL cells to antibody-induced apoptosis, unless they express high levels of Mcl-1. In contrast, combined treatment with rituximab and ABT-737 failed to further enhance apoptosis in rituximab-sensitive B-NHL cells (Figure S3). Pharmacomimetics of the BH3-only protein Noxa, a physiologic antagonist of Mcl-1, might be effective to overcome apoptosis resistance in B-NHL cells overexpressing Mcl-1.38-41
The BH3-mimetic ABT-737 sensitizes B-NHL cells expressing high levels of Bcl-2 and Bcl-xL to rituximab-induced apoptosis. (A) Resistant B-NHL cells were incubated for 48 hours with cross-linked rituximab (Rc), the pharmacologic BH3-mimetic ABT-737 (100 nM for Jeko-1 and HT cells, 5 nM for Sc-1 cells), or both. The fraction of cells with apoptotic DNA fragmentation was quantified flow cytometrically; mean values plus SD of 3 independent experiments are shown. (B) Resistant B-NHL cells were incubated for 24 hours with staurosporine (STS, 25 nM), the pharmacologic BH3-mimetic ABT-737 (100 nM for Jeko-1 and HT cells, 5 nM for Sc-1 cells), or both. The fraction of cells with apoptotic DNA fragmentation was quantified flow cytometrically; mean values plus SD of 3 independent experiments are shown.
The BH3-mimetic ABT-737 sensitizes B-NHL cells expressing high levels of Bcl-2 and Bcl-xL to rituximab-induced apoptosis. (A) Resistant B-NHL cells were incubated for 48 hours with cross-linked rituximab (Rc), the pharmacologic BH3-mimetic ABT-737 (100 nM for Jeko-1 and HT cells, 5 nM for Sc-1 cells), or both. The fraction of cells with apoptotic DNA fragmentation was quantified flow cytometrically; mean values plus SD of 3 independent experiments are shown. (B) Resistant B-NHL cells were incubated for 24 hours with staurosporine (STS, 25 nM), the pharmacologic BH3-mimetic ABT-737 (100 nM for Jeko-1 and HT cells, 5 nM for Sc-1 cells), or both. The fraction of cells with apoptotic DNA fragmentation was quantified flow cytometrically; mean values plus SD of 3 independent experiments are shown.
Targeting PI3K signaling overcomes rituximab resistance mediated by Mcl-1 in vitro and in vivo
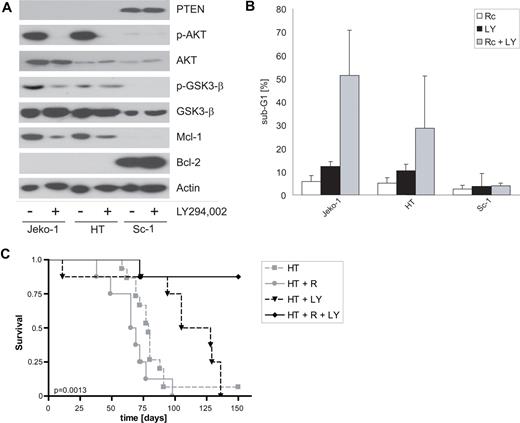
As an alternative strategy to sensitize Jeko-1 and HT B-NHL cells, we studied the pharmacologic modulation of upstream regulators of Mcl-1 expression. Several mechanisms of posttranscriptional regulation of Mcl-1 have been described, some of which involve the PI3K–Akt signaling pathway.42,43 In the present study, high endogenous Mcl-1 expression in rituximab-resistant Jeko-1 and HT cells correlated with active PI3K-Akt signaling, as demonstrated by constitutive phosphorylation of Akt and Akt downstream targets, such as glycogen synthase kinase 3-β (GSK3-β). Moreover, those B-NHL cell lines had lost expression of the Phosphatase and Tensin homolog deleted in chromosome 10 (PTEN) tumor suppressor, a negative regulator of PI3K (Figure 5A). Treating Jeko-1 and HT cells with pharmacologic inhibitors of PI3K, such as LY294,002 or wortmannin, effectively reduced endogenous expression of antiapoptotic Mcl-1 (Figure 5A). Moreover, PI3K inhibitors sensitized Jeko-1 and HT, but not Sc-1 B-NHL cells to rituximab-induced apoptosis (Figure 5B).
Pharmacologic inhibition of PI3K signaling reduces Mcl-1 expression and sensitizes B-NHL cells to rituximab-induced apoptosis in vitro and rituximab treatment in vivo. (A) Immunoblot analyses of rituximab-resistant B-NHL cells treated with the PI3K inhibitor LY294,002 (10 μM) or vehicle (−) using the indicated primary antibodies. Note the down-regulation of endogenous Mcl-1 expression in PTEN-negative Jeko-1 and HT B-NHL cells by the PI3K inhibitor. (B) Rituximab-resistant B-NHL cells were incubated for 48 hours with cross-linked rituximab (Rc), the PI3K inhibitor LY294,002 (LY, 10 μM), or both. The fraction of cells with apoptotic DNA fragmentation was quantified flow cytometrically; mean values plus SD of 3 independent experiments are shown. (C) Kaplan-Meier plots of symptom-free survival of NOD/SCID mice after intravenous inoculation of 107 HT cells. Starting on day 5, 2 groups of mice received intraperitoneal injections of rituximab (HT + R, gray dots, solid line) or vehicle (HT, gray boxes, dashed line). A third group received intraperitoneal injections of the PI3K inhibitor LY294,002 (HT + LY, black triangles, dashed line), whereas the fourth group was treated with LY294,002 in combination with rituximab (HT + R + LY, black diamonds, solid line); 8 mice were treated in each group. Note that LY294,002 successfully sensitized the mice toward rituximab treatment.
Pharmacologic inhibition of PI3K signaling reduces Mcl-1 expression and sensitizes B-NHL cells to rituximab-induced apoptosis in vitro and rituximab treatment in vivo. (A) Immunoblot analyses of rituximab-resistant B-NHL cells treated with the PI3K inhibitor LY294,002 (10 μM) or vehicle (−) using the indicated primary antibodies. Note the down-regulation of endogenous Mcl-1 expression in PTEN-negative Jeko-1 and HT B-NHL cells by the PI3K inhibitor. (B) Rituximab-resistant B-NHL cells were incubated for 48 hours with cross-linked rituximab (Rc), the PI3K inhibitor LY294,002 (LY, 10 μM), or both. The fraction of cells with apoptotic DNA fragmentation was quantified flow cytometrically; mean values plus SD of 3 independent experiments are shown. (C) Kaplan-Meier plots of symptom-free survival of NOD/SCID mice after intravenous inoculation of 107 HT cells. Starting on day 5, 2 groups of mice received intraperitoneal injections of rituximab (HT + R, gray dots, solid line) or vehicle (HT, gray boxes, dashed line). A third group received intraperitoneal injections of the PI3K inhibitor LY294,002 (HT + LY, black triangles, dashed line), whereas the fourth group was treated with LY294,002 in combination with rituximab (HT + R + LY, black diamonds, solid line); 8 mice were treated in each group. Note that LY294,002 successfully sensitized the mice toward rituximab treatment.
To validate this strategy to reverse endogenous rituximab resistance by targeted pharmacotherapy in vivo, we established a xenograft model of PTEN-deficient HT B-NHL cells in irradiated NOD/SCID mice. The onset of tumor symptoms in HT-grafted mice occurred much later than in mice grafted with Ramos cells (Figures 2D, 5C, S2). In keeping with resistance to rituximab-induced apoptosis observed in vitro (Figure 1), rituximab treatment failed to modulate the course of HT-grafted mice (Figure 5C). In contrast, combining rituximab with the PI3K inhibitor LY294,002 significantly (P < .009, log rank test) prolonged survival of HT-grafted NOD/SCID mice, suggesting that down-modulation of the PI3K-Akt signaling pathway could be a successful strategy to sensitize B-NHL cells to antibody treatment in vivo (Figure 5C). Interestingly, treatment with LY294,002 alone was also effective in our preclinical model, but clearly to a much lesser extent than combination therapy with rituximab (Figure 5C). Taken together, pharmacologic modulation of aberrant PI3K-Akt signaling successfully overcame intrinsic resistance of B-NHL cells to rituximab-induced apoptosis in vitro and in vivo.
Expression pattern of Bcl-2 family proteins correlates with clinical outcome after rituximab-based chemoimmunotherapy for relapsed lymphoma
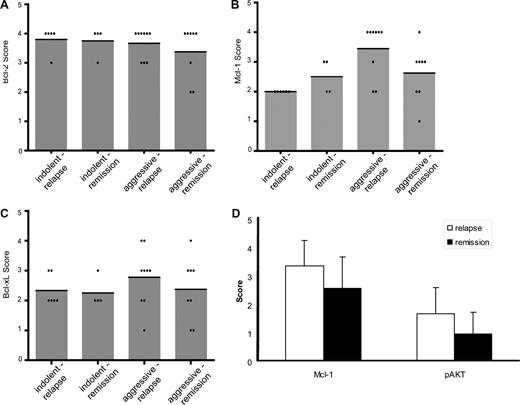
To study whether the expression of antiapoptotic Bcl-2 family proteins might correlate with the clinical outcome of B-NHL patients after treatment with rituximab, we performed exploratory analyses in surplus tumor biopsies from patients treated within phase 2 studies of rituximab-based salvage therapy for relapsed indolent or aggressive lymphoma.11 This population was selected because all patients were uniformly treated (a substantial fraction being rituximab-naive) and had consented in the participation of a scientific protocol. Paraffin-embedded tumor samples were available for 14 patients with indolent lymphomas (most frequently FL) and 21 patients with aggressive lymphomas (mostly DLBCL). Patients were separated into 2 groups: those patients who relapsed after rituximab-based salvage treatment including high-dose therapy with autologous stem cell support and those patients who remained in remission. Whereas the majority of indolent and aggressive lymphomas exhibited high protein expression for Bcl-2 (Figure 6A), thus precluding a meaningful correlation with clinical outcome, relapsing aggressive lymphomas tended to express higher levels of antiapoptotic Mcl-1 (Figure 6B) and Bcl-xL (Figure 6C). Because of small sample size, none of the comparisons reached statistical significance. However, a P value of .08 was calculated for the difference in Mcl-1 expression between patients with aggressive lymphoma experiencing a second relapse and those remaining in second remission (Figure 6B). Moreover, 80% (4 of 5) of patients with aggressive lymphoma and high Mcl-1 expression relapsed, whereas 70% (7 of 10) of patients with aggressive lymphoma and low Mcl-1 expression remained in second remission. Interestingly, the increase in Mcl-1 expression also correlated with enhanced activation of Akt in relapsing lymphomas (Figure 6D). These results might indicate a trend toward inferior clinical outcome after rituximab-based therapy of aggressive lymphomas with high endogenous Mcl-1 expression, which could be amendable by PI3K/Akt-directed pharmacotherapies.
Correlation of clinical outcome after rituximab-based chemoimmunotherapy with the expression pattern of Bcl-2 family proteins. Immunohistochemical analysis of expression of Bcl-2 (A), Mcl-1 (B), and Bcl-xL (C) in tumor biopsies of 14 patients with indolent B-cell lymphomas and 21 patients with aggressive B-cell lymphomas. Patients were grouped according to histology and clinical course (relapse or continued remission after rituximab-based salvage therapy). Protein expression was quantified after the IRS scoring system, and individual scores (points) as well as mean scores ( ) are given. (D) For patients with aggressive lymphomas, the expression of Mcl-1 and phosphorylated AKTS473 (p-AKT) was analyzed in relation to clinical course (mean values + SD).
) are given. (D) For patients with aggressive lymphomas, the expression of Mcl-1 and phosphorylated AKTS473 (p-AKT) was analyzed in relation to clinical course (mean values + SD).
Correlation of clinical outcome after rituximab-based chemoimmunotherapy with the expression pattern of Bcl-2 family proteins. Immunohistochemical analysis of expression of Bcl-2 (A), Mcl-1 (B), and Bcl-xL (C) in tumor biopsies of 14 patients with indolent B-cell lymphomas and 21 patients with aggressive B-cell lymphomas. Patients were grouped according to histology and clinical course (relapse or continued remission after rituximab-based salvage therapy). Protein expression was quantified after the IRS scoring system, and individual scores (points) as well as mean scores ( ) are given. (D) For patients with aggressive lymphomas, the expression of Mcl-1 and phosphorylated AKTS473 (p-AKT) was analyzed in relation to clinical course (mean values + SD).
) are given. (D) For patients with aggressive lymphomas, the expression of Mcl-1 and phosphorylated AKTS473 (p-AKT) was analyzed in relation to clinical course (mean values + SD).
Discussion
Cell-intrinsic resistance mechanisms are regarded as major determinants of the response to cytotoxic cancer therapies, such as DNA-damaging agents and radiation. Resistant phenotypes are either selected during oncogenic transformation and tumor progression, which both require the abrogation of important tumor suppressive mechanisms, such as apoptosis, cell-cycle arrest, and senescence. These effector functions are regulated by signal transduction pathways, which equally mediate the effects of various anticancer agents. Accordingly, transformed cells may harbor primary resistance to cytotoxic therapies.44,45 Moreover, secondary resistance may evolve under the selective pressure of anticancer therapies. These comprise the up-regulation of efflux pumps, such as Mdr-1, or prosurvival factors.46
In contrast, immune escape of cancer is mainly attributed to indirect mechanisms, such as the secretion of immunosuppressive factors, the down-modulation of the antigen-presentation machinery or the expression of death ligands, which kill tumor-infiltrating lymphocytes.47,48 More recently, cell-intrinsic resistance mechanisms were identified, which modulate the susceptibility of cancers to cellular immunotherapy in vitro and in vivo.22,49-53 These studies have established a molecular basis for the phenomenon of “cross-resistance” against cytotoxic and immunologic anticancer therapies. In line with this concept, it is of particular interest to assess whether such cancer cell-intrinsic resistance mechanisms are also in place to determine the efficacy of therapeutic antibodies, which are frequently combined with cytotoxic agents for treatment of cancer patients.
The chimeric monoclonal antibody rituximab is paradigmatic for the successful clinical application of adoptive cancer immunotherapy. As the physiologic role of its target, the CD20 membrane antigen, is largely unknown, and CD20-deficient mice fail to exhibit a significant developmental or functional B-cell phenotype,54,55 rituximab's clinical activity must rely on direct or indirect cytotoxic effects. This contrasts therapeutic antibodies, such as trastuzumab, bevacizumab, cetuximab, or panitumumab, which are thought to act as modulators of signal transduction events rather than or in addition to CDC or ADCC. Accordingly, the susceptibility of neoplastic B cells to rituximab should be determined by the expression of the target antigen, as well as the presence of a functional complement system and/or macrophages and NK cells of the host. However, resistance to rituximab may occur in CD20-positive lymphoma13,20 and in the absence of immune or complement dysfunction. Hence, additional factors might impact on the rituximab response of B-NHL cells.
Recently, Jazirehi et al56 characterized clones derived from the Ramos and Daudi Burkitt lymphoma cell lines that were selected for secondary resistance by continuous exposure to increasing concentrations of rituximab. They found that these clones resisted chemosensitization as well as direct induction of apoptosis by rituximab, which was explained by the antibody's inability to shut down signal transduction via MAPK and NF-κB pathways.56 This was in line with previous reports from the same group, which suggested negative regulation of survival pathways as the main mechanism for direct chemosensitization of B-NHL cells by rituximab.57-60 Moreover, using a similar model of secondary rituximab resistance, Czuczman et al described global downmodulation of CD20 expression as well as loss of proapoptotic Bax and Bak as acquired escape mechanisms in response to prolonged selection with rituximab.34,61 Contrasting these studies, we have analyzed primary sensitivity and resistance of B-NHL cells to induction of apoptosis by rituximab. Accordingly, resistance mechanisms described and validated in our model did not evolve in response to prior rituximab exposure but were constitutively present in the B-NHL cells. Thus, our model primarily aimed to reflect primary resistance to rituximab.
In our study, the monomeric antibody exhibited marginal cytotoxic activity in vitro, whereas cross-linked rituximab elicited a strong apoptotic response in some B-NHL cell lines. Interestingly, rituximab-induced apoptosis proceeded via the “mitochondrial” pathway of caspase activation, which is regulated by the Bcl-2 family of proteins. The significance of our observation could be corroborated by a murine xenograft model, which demonstrated that enforced expression of an antiapoptotic Bcl-2 protein abrogated the direct therapeutic activity of rituximab in vivo. Moreover, exploratory analyses of tumor biopsies from rituximab-treated patients suggested a correlation between high expression levels of antiapoptotic Mcl-1 and Bcl-xL with inferior clinical outcome in aggressive lymphomas.
Molecular characterization of constitutively resistant B-NHL cells revealed Bcl-2 plus Bcl-xL, as well as PI3K-dependent up-regulation of Mcl-1 as major determinants of sensitivity to apoptosis induced by rituximab. Pharmacologic targeting of these factors successfully sensitized endogenously resistant B-NHL cells to antibody-mediated apoptosis, thus confirming their potential suitability as targets for clinical resistance modulation. Importantly, a pharmacologic PI3K inhibitor successfully reversed rituximab resistance of lymphoma-bearing mice in vivo. This strategy is supported by recent findings in artificially selected rituximab-resistant clones, which also exhibited increased MAPK, PI3K, and NF-κB signaling resulting in expression of Bcl-2, Bcl-xL, and Mcl-1,56 and studies of sensitization of cancer cells by siRNA-mediated down-regulation of Mcl-1 or Bfl-1.62,63
Currently, it remains unclear whether rituximab's direct activities primarily target survival signal transduction pathways to down-regulate antiapoptotic proteins19 or whether growth factor signaling pathways and the expression pattern of Bcl-2 proteins determine the cell intrinsic-sensitivity to rituximab, as shown in the present study and by others.34 The latter concept is in keeping with recent work on the role of the so-called BH3-only members of the Bcl-2 family as determinants of drug-sensitivity in B-NHL cells.41,64 In summary, rituximab exerts a direct proapoptotic activity on B-NHL cells via the “mitochondrial” pathway of caspase activation. Functional defects in this pathway determine antibody resistance in vitro and in vivo, which can be reversed by molecularly targeted pharmacologic interventions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Abbott Laboratories and G. P. Nolan for providing reagents, A. Hohberger and A. Konur for help with experiments, and Ch. Huber for support and advice.
This work was supported by the Wilhelm Sander-Stiftung (grant 2005.136.1-2) (M.S.), Roche (G.H.), and the Immunology Cluster of Excellence Rheinland-Pfalz. C.S. was an affiliated graduate student of the GK1043 of the Deutsche Forschungsgemeinschaft.
Authorship
Contribution: C.S., G.H., P.S.H., F.G., and S.H. carried out research; C.S., G.H., P.S.H., F.G., and K.W.S. reviewed and analyzed data and reviewed the manuscript; and M.S. supervised the project, designed research, reviewed and analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin Schuler, Department of Medicine (Cancer Research), West German Cancer Center, University Hospital Essen, Hufelandstrasse 55, 45147 Essen, Germany; e-mail: martin.schuler@uk-essen.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal