Abstract
Activin A, BMP2, and BMP4, 3 members of the transforming growth factor-β family, are involved in the regulation of hematopoiesis. Here, we explored the role of these molecules in human megakaryopoiesis using an in vitro serum-free assay. Our results highlight for the first time that, in the absence of thrombopoietin, BMP4 is able to induce CD34+ progenitor differentiation into megakaryocytes through all stages. Although we have previously shown that activin A and BMP2 are involved in erythropoietic commitment, these molecules have no effect on human megakaryopoietic engagement and differentiation. Using signaling pathway-specific inhibitors, we show that BMP4, like thrombopoietin, exerts its effects on human megakaryopoiesis through the JAK/STAT and mTor pathways. Inhibition of the BMP signaling pathway with blocking antibodies, natural soluble inhibitors (FLRG or follistatin), or soluble BMP receptors reveals that thrombopoietin uses the BMP4 pathway to induce megakaryopoiesis, whereas the inverse is not occurring. Finally, we show that thrombopoietin up-regulates the BMP4 autocrine loop in megakaryocytic progenitors by inducing their production of BMP4 and up-regulating BMP receptor expression. In summary, this work indicates that BMP4 plays an important role in the control of human megakaryopoiesis.
Introduction
The production of mature circulating blood cells from stem cells involves a series of successive differentiation steps that progressively decrease stem cell developmental and proliferative capacities. This process is governed by many specific hematopoietic cytokines and by interactions of cells with their environment.1 Megakaryocytes (MKs) derive from the commitment of undifferentiated pluripotent stem cells into a lineage that results in the terminal differentiation of mature platelets.2 Although erythroid and MK lineages are likely to derive from a common progenitor, signals that regulate the ultimate separation of these lineages are not clearly identified. However, we have previously described that members of the transforming growth factor-β (TGF-β) family are involved in human erythropoiesis.3
The TGF-β family, including activins and bone morphogenetic proteins (BMPs), regulates numerous cellular events, such as proliferation, differentiation, migration, adhesion, and apoptosis. Whereas TGF-β is one of the important regulatory elements of the hematopoietic system, activin A and BMP also play a role in hematopoiesis at various stages of cell differentiation.4-8 We have shown that activin A and BMP2 induce cell commitment and differentiation toward erythropoiesis, even in the absence of erythropoietin (EPO). Their biologic activities are antagonized by binding with follistatin or FLRG (follistatin-related gene), 2 secreted glycoproteins expressed by human bone marrow and regulated by TGF-β and activin A.3,9 FLRG and follistatin are involved in the regulation of human hematopoietic cell adhesiveness in immature hematopoietic progenitors and stem cells through direct interactions between the type I motifs of fibronectin and follistatin domains,10 constituting a new mechanism of human hematopoiesis regulation.9
The involvement of BMP molecules in hematopoiesis at various stages of stem cell differentiation has been largely documented during the past decade.8,11-15 During embryogenesis, BMP molecules play a critical role in the formation of hematopoietic and endothelial precursors emerging from the ventral mesoderm.16 In particular, in Xenopus embryos, ectopic BMP4 expression induces several hematopoietic genes.11,16 In humans, BMP2, BMP4, and BMP7 regulate the proliferation, maintenance,17 clonogenicity, and repopulating capacity of CD34+CD38− primitive hematopoietic populations.12 BMP2 and BMP4, either alone or in combination with activin A, regulate erythropoiesis in various models.9
Out of the BMP family, BMP4 emerges as a key player in the regulation of stem cell biology, such as in the stem cell fate decision reported in various tissues and species.18,19 In vivo, homozygous BMP4-deleted mice display a lethal phenotype characterized by a reduced extraembryonic mesoderm, including blood islands.20 In mammalian models, BMP4 is involved in the hematopoietic differentiation of embryonic stem cells15,21,22 and the induction of blood lineages from human skeletal or neural tissues.8 It now becomes clear that BMP4 stands out of the TGFβ family as a key stem cell regulator in both normal and malignant contexts.23
MKs generate platelets that play an essential role in thrombosis and hemostasis. Although numerous hematopoietic growth factors regulate different aspects of MK biology, thrombopoietin (TPO), the ligand of c-mpl, has been identified as a central regulator at all stages of MK development.24,25 In this study, we used an in vitro differentiation system to reproduce the different stages of human megakaryopoiesis to explore the role of activin A, BMP2, and BMP4 in MK differentiation. Our data show that these molecules are not equally involved in human MK regulation, and we highlight for the first time the role of BMP4 as an important regulator of MK cells. Contrary to the other cytokines explored, BMP4 alone is able to regulate the main stages of human megakaryopoiesis and is involved in the control of megakaryopoiesis by TPO.
Methods
Proteins, antibodies, and chemicals
Human TPO (PeproTech, Rocky Hill, NJ), activin, BMP2, BMP4, FLRG, follistatin (50 ng/mL), and up to 4 μg/mL sBMPR-Ia or sBMPR-Ib (R&D Systems, Minneapolis, MN) were used as indicated and following the manufacturers' instructions. Blocking experiments were performed according to the manufacturer's instructions (R&D Systems) using goat anti–TPO-R and its isotype control goat IgG (20 μg/mL) or monoclonal mouse anti-BMP4 and its isotype control mouse IgG2b (1 μg/mL). Analysis of signaling pathway inhibitors was performed using the protein-kinase inhibitors AG490 (25 μM), PD98059 (2 μM), and LY294002 (10 μM) or rapamycin (100 nM; Sigma-Aldrich, St Louis, MO). Dimethyl sulfoxide had no effect by itself as described.26
Primary cells and culture conditions
Normal bone marrow samples were obtained from healthy consenting donors for allogeneic transplant. Mononuclear cells, separated by a Ficoll gradient, were subjected to CD34 immunomagnetic separation (Invitrogen, Carlsbad, CA). Megakaryopoiesis was obtained by modifying described procedures.27 Briefly, CD34+ cells (6 × 105 cells/mL) were cultured in serum-free Iscove modified Dulbecco medium (IMDM; Invitrogen) in the presence of 15% BIT (BSA, insulin, transferrin; StemCell Technologies, Vancouver, BC) supplemented with 10 ng/mL interleukin-6 (IL-6), 50 ng/mL stem cell factor (SCF), 10 ng/mL IL-11, and 10 ng/mL IL-3 (PeproTech). This serum-free medium (referred to as 4GF, growth factor) constitutes the basal medium that we used in all experiments except the long-term culture-initiating cells (LTC-IC) assay. In the LTC-IC assay, CD34+CD38− cells,10 further purified by a FACVantageSE cell sorter (BD Biosciences, San Jose, CA), were incubated (2 × 103 cells/mL) in IMDM serum-free medium supplemented with 15% BIT and cytokines used alone or in combination with cytokines, such as 100 ng/mL Flt3-L (PeproTech). After 10 days, cells were harvested, washed, and plated in the LTC-IC assay. Early erythropoiesis was obtained as described.3
Cell and platelet counting, cell viability
Viable cell numbers were obtained by trypan blue counting on a Malassez cell. The fold proliferation represented the ratio between counts at day 3 and number of input cells. Platelets were numbered using a Thomas cell counting chamber.
Measurement of surface glycoproteins and ploidy
The cells were incubated for 30 minutes at 4°C with 0.1 μg antibody/106 cells (anti-CD34, anti-CD41, anti-CD61, anti-CD36 [Dako France, Trappes, France], anti–glycophorin-A [GPA; Invitrogen], anti-CD42a, and anti-CD42b [Beckman Coulter, Villepinte, France]). Conjugated irrelevant isotype-matched controls were used to assess nonspecific fluorescence. Cell DNA content was measured by staining with 50 μg/mL propidium iodide as described.28 Flow cytometric analysis was performed using a Facscalibur cell analyzer (Becton Dickinson France, Le Pont de Claix, France) on CD41+ gated cells.
Progenitor assays
The total number of colony-forming cells (CFCs) was determined by plating into Iscove methylcellulose medium (StemCell Technologies), and colonies were counted in situ after 16 days.3 The number of CFCs and MK progenitors (CFU-MK) was determined following the manufacturer's instructions using a collagen medium containing TPO, IL-6, and IL-3. Megakaryocytic (pink) and nonmegakaryocytic (blue) colonies were counted after 10 to 12 days in situ using the defined scoring criteria (StemCell Technologies).
LTC-IC assays
After 10 days of culture, all cells in each well were cocultured with murine MS5 cell feeder (Dr Coulombel, Hôpital Paul Brousse, Villejuif. France) in human long-term culture medium (StemCell Technologies) supplemented with freshly dissolved 10−6 M hydrocortisone sodium hemisuccinate (Sigma-Aldrich), with weekly half-medium change.10 After 5 weeks, both nonadherent and adherent cells were harvested, pooled, and washed; and the number of CFCs produced was expressed as W5-CFC/1000 initial CD34+CD38− cells and correlated with the number of primitive progenitors in the original input suspension.
RNA isolation and analysis
Total cellular RNA was isolated from samples lysed in TRI REAGENT (Sigma-Aldrich) and analyzed by reverse-transcribed polymerase chain reaction (RT-PCR) as we previously described3 using specific gene primer (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) simultaneously with GAPDH, used as internal control. For quantitative RT-PCRq, 1 μg of RNA, extracted using a mini-RNA extraction kit (Qiagen, Valencia, CA), was retro-transcribed with Superscript II enzyme (Invitrogen) and amplified by PCR reactions run in duplicate on a Real-Time PCR system (Lightcycler; Roche Diagnostics France, Meylan, France) and normalized to TBP and COF gene expression. Primer sequences are described in Table S2.
Western blot analysis
Cell lysates were prepared and quantified as described,3 and 10 μg protein lysate per lane was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto membranes (Millipore France, Molsheim, France). For platelet factor 4 (PF4) secretion, the culture supernatant was harvested after 10 to 18 days, and 30 μL cleared supernatant (10 minutes at 3000g) was separated and transferred onto membranes. Human platelet extracts were used as a positive control for PF4 detection (not shown). Filters were incubated with anti-PF4 (Dako France) antiactin (Roche Diagnostics) or anti-CD42a (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies, then incubated with their relevant peroxidase-conjugated secondary antibody (Dako) and revealed with an enhanced chemiluminescence detection kit (Amersham Biosciences France, Orsay, France).
BMP4 and PF4 quantification
The supernatant of cultured cells (6 × 105cells/mL) was harvested after 3 to 18 days and cleared. Various dilutions of this processed supernatant were placed in 96-well plates (100 μL/well) to quantify proteins using the human BMP4-ELISA (Ray Biotech France, Le Perray en Yvelines, France) or PF4-ELISA (R&D Systems) kits following the manufacturer's instructions. Measures were done in triplicate and repeated several times for the same experiment. The optical density at 450 nm was determined using a microplate reader (Dynex MRX; Dynex Technologies, Worthing, United Kingdom).
Statistics
Differences in the various treatment groups were assessed using the paired Student t test. Significant P values (< .05) identify significant effects compared with nontreated cells.
Results
Both BMP4 and TPO, but not activin A and BMP2, increase MK cell surface markers
We have established that only activin A and BMP2 alone can regulate human erythropoiesis in vitro,3 and we therefore pursued the characterization of BMP4 activities in other aspects of hematopoiesis. To study the role of these molecules in human megakaryopoiesis, we used purified primary CD34+ bone marrow progenitors to generate an accurate in vitro serum-free MK differentiation model, representing the major steps of MK maturation (expression of MK specific markers, genes, and proteins, Figure S1). In these conditions, we observed a rapid increase of human FOG-1 and FOG-2 expression, but FOG-2 expression seemed more pronounced in MK cells (Figure S1D; Table S3). As the different receptors for activin and BMP are expressed on CD34+ cells (Figures S1E, S2A) and at various stages of the culture (Figure S1E), these molecules have the potential to regulate MK cells, as suggested.29
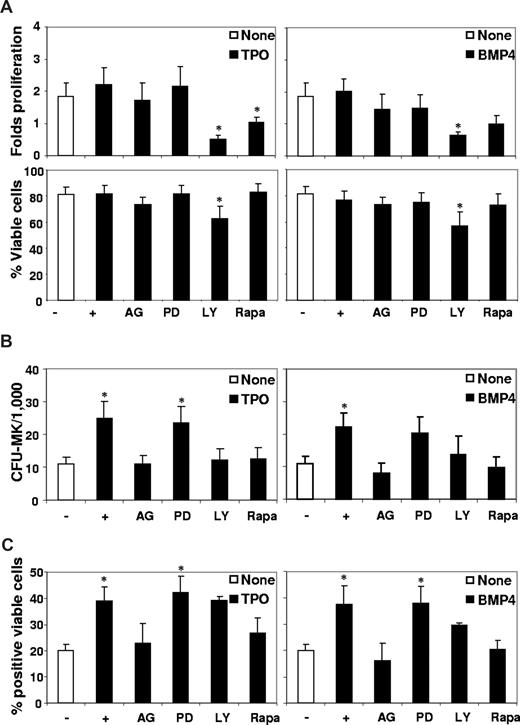
We first assayed the effects of TPO, activin A, BMP2, and BMP4, alone or in combination, on the proliferation, viability, and surface marker expression of primary CD34+ progenitors placed in the MK system. Cell proliferation and viability determination after 3 days of culture showed no significant effect of any molecule (Figure 1A). Flow cytometric analysis of MK surface markers expressed on viable cells after 7 days of culture indicated that only TPO and BMP4 significantly enhanced the expression of the early CD41 (P = .001 and .01, respectively) and CD61 (P = .002 and .05, respectively) and of the late CD42a (P = .001 and .019, respectively) and CD42b (P = .05 and .013, respectively) MK markers (Figure 1B). Kinetic analysis of CD41 and CD42 expression throughout the culture indicated that TPO or BMP4 induce a 2-fold increased expression of these markers, suggesting that they induce a true MK differentiation (Figure S2B). Simultaneous addition of TPO and BMP4 did not elicit a more potent MK differentiation response than cytokines alone, as similar numbers of antigen marker–positive cells were obtained for most surface markers. In comparison, no significant effect was observed on any surface marker by treating the cells with either activin A or BMP2 (Figure 1B). These results indicate that none of these molecules significantly affects balanced apoptosis, by acting as a surviving factor, for instance, or induces the expansion of primary cells. Inversely, as suggested by a specific MK marker increase, BMP4 seems to exert a significant effect on MK lineage, to the same extent as TPO.
Effects of TPO, activin (ACT), BMP2, and BMP4 on the proliferation, viability, and cell surface marker expression of primary CD34+ progenitors, on primitive, total, and MK progenitors. Bone marrow CD34+ cells (6 × 105/mL) were incubated in 4GF serum-free medium for 3 to 7 days in the presence or not (None) of 50 ng/mL TPO, activin A (ACT), BMP2, or BMP4. (A) After 3 days of culture, cell proliferation and viability were evaluated by trypan blue counting. Fold proliferation was determined by reference to the number of input viable cells. Results represent the mean plus or minus SEM of 24 experiments for addition of TPO or BMP4 and 12 experiments for activin or BMP2. (B) Phenotypic analysis of the hematopoietic progenitor CD34, the erythroid-specific marker glycophorin A (GPA), early MK-specific markers CD41 and CD61, and late MK markers CD42a and CD42b was performed by flow cytometric analysis using a Facscalibur cell analyzer (Beckman Coulter) and gating on viable cells. Results are presented as percentages of positive viable cells plus or minus SEM of 14 to 22 experiments for addition of TPO or BMP4 and 8 experiments for activin or BMP2. (C) The CFC or CFU-MK content of treated cells was analyzed, and results, expressed as CFC/1000 or CFU-MK/1000 seeded cells, represent the mean value on day 3 plus or minus SEM of, respectively, 14, 8, or 5 experiments for TPO/BMP4 alone, TPO + BMP4, and ACT or BMP2, and the mean value on day 7 plus or minus SEM of 8 experiments for all conditions. (D) Further sorted CD34+CD38− cells were incubated (2 × 103/mL) in 96-well round-bottom plates in strict serum-free IMDM containing 15% BIT without any cytokines (0) or with SCF (100 ng/mL), IL-3 (20 ng/mL), Flt3L (100 ng/mL), or TPO (50 ng/mL) alone ( ) or in combination with BMP4 (50 ng/mL; ■). After 10 days, the cells were placed in an LTC-IC assay. Results are presented on a log scale graph as week 5-CFC/1000 input cells plus or minus SEM of 5 experiments. * indicates statistically significant difference from those in nontreated cells (P < .05).
) or in combination with BMP4 (50 ng/mL; ■). After 10 days, the cells were placed in an LTC-IC assay. Results are presented on a log scale graph as week 5-CFC/1000 input cells plus or minus SEM of 5 experiments. * indicates statistically significant difference from those in nontreated cells (P < .05).
Effects of TPO, activin (ACT), BMP2, and BMP4 on the proliferation, viability, and cell surface marker expression of primary CD34+ progenitors, on primitive, total, and MK progenitors. Bone marrow CD34+ cells (6 × 105/mL) were incubated in 4GF serum-free medium for 3 to 7 days in the presence or not (None) of 50 ng/mL TPO, activin A (ACT), BMP2, or BMP4. (A) After 3 days of culture, cell proliferation and viability were evaluated by trypan blue counting. Fold proliferation was determined by reference to the number of input viable cells. Results represent the mean plus or minus SEM of 24 experiments for addition of TPO or BMP4 and 12 experiments for activin or BMP2. (B) Phenotypic analysis of the hematopoietic progenitor CD34, the erythroid-specific marker glycophorin A (GPA), early MK-specific markers CD41 and CD61, and late MK markers CD42a and CD42b was performed by flow cytometric analysis using a Facscalibur cell analyzer (Beckman Coulter) and gating on viable cells. Results are presented as percentages of positive viable cells plus or minus SEM of 14 to 22 experiments for addition of TPO or BMP4 and 8 experiments for activin or BMP2. (C) The CFC or CFU-MK content of treated cells was analyzed, and results, expressed as CFC/1000 or CFU-MK/1000 seeded cells, represent the mean value on day 3 plus or minus SEM of, respectively, 14, 8, or 5 experiments for TPO/BMP4 alone, TPO + BMP4, and ACT or BMP2, and the mean value on day 7 plus or minus SEM of 8 experiments for all conditions. (D) Further sorted CD34+CD38− cells were incubated (2 × 103/mL) in 96-well round-bottom plates in strict serum-free IMDM containing 15% BIT without any cytokines (0) or with SCF (100 ng/mL), IL-3 (20 ng/mL), Flt3L (100 ng/mL), or TPO (50 ng/mL) alone ( ) or in combination with BMP4 (50 ng/mL; ■). After 10 days, the cells were placed in an LTC-IC assay. Results are presented on a log scale graph as week 5-CFC/1000 input cells plus or minus SEM of 5 experiments. * indicates statistically significant difference from those in nontreated cells (P < .05).
) or in combination with BMP4 (50 ng/mL; ■). After 10 days, the cells were placed in an LTC-IC assay. Results are presented on a log scale graph as week 5-CFC/1000 input cells plus or minus SEM of 5 experiments. * indicates statistically significant difference from those in nontreated cells (P < .05).
Effects of BMP4 on human hematopoietic progenitors
We evaluated the content in total CFU-MK of TPO- or BMP4-treated CD34+ progenitors. As expected, although TPO alone had little effect on total CFC colonies after 3 or 7 days (Figure 1C), it rapidly increased the number of CFU-MK (2.6-fold after 3 days, P = .001). Similarly, BMP4 alone had no or little effect on total CFC but significantly increased the number of CFU-MK (2.5-fold, P = .002) after 3 days of culture. Whereas the number of CFU-MK detected in TPO-treated cells at day 7 was not significantly different from that in untreated cells, BMP4-treated cells still displayed a significantly higher CFU-MK content (1.9-fold, P = .016) than untreated cells. No cooperation between TPO and BMP4 was observed on either total CFC or CFU-MK progenitors at any time of the culture. Although, as expected,3 only activin A significantly increased the number of total CFC detected (P = .032), neither activin A nor BMP2 showed any effect on the CFU-MK compartment, suggesting that they are not directly involved in MK cells (Figure 1C). We compared the effects of the various proteins on CFC adhesion as involved in early and late MK regulation,30 and observed that only TPO and BMP4 increased the number of adherent CFC (Figure S2C).
We then screened for BMP4 partners on very primitive progenitors, which implied to perform the experiment in strict serum-free conditions, without our usual 4GF combination. Sorted CD34+CD38− cells were incubated with or without BMP4 in the presence or not of TPO, IL-3, SCF, and Flt3-L, used alone or in combination. After 10 days, wells were evaluated for their primitive hematopoietic progenitor content using an LTC-IC assay, and the number of CFCs produced was expressed as W5-CFC/1000 initial CD34+CD38− cells. Results indicate that BMP4, IL3, FLt3L, and TPO alone, but not SCF, amplified primitive progenitors. BMP4 cooperated with IL-3 (7-fold) or TPO (2-fold) to amplify W5-CFC, whereas it slightly decreased the effect of Flt3-L (0.6-fold) and of the TPO/SCF or TPO/Flt3L combinations (0.5-fold and 0.9-fold, respectively; Figure 1C). Interestingly, BMP4 strongly synergized with SCF (50-fold increase over SCF alone) to amplify progenitors. Our data underline the cooperation of BMP4 with SCF and IL-3 (both involved in MK regulation27 ) and the absence of synergistic effect with TPO in the modulation of primitive progenitors.
Altogether, these results indicate that BMP4, like TPO, amplifies very primitive hematopoietic progenitors and induces their adhesion and engagement toward MK cells differently from the other members of the TGF-β family. Conversely to TPO, BMP4 seems capable of maintaining a large pool of MK progenitors throughout time.
Effects of activin A, BMP2, and BMP4 on late stages of human megakaryopoieis
A unique feature of MK cells is that they undergo an endomitotic cell cycle, resulting in a polyploidized phenotype. We analyzed on CD41+-gated cells the DNA content of TPO- or BMP4-treated cells after 3, 7, 10, or 18 days (Table 1). Both TPO and BMP4 increase a ploidization of CD34+ cells as indicated by, respectively, up to 22% at the 4N ploidy level and to 6% at the 8N ploidy level compared with a maximum of 14% at the 4N level and 3% at the 8N level in untreated cells. The effects of BMP4 and TPO appear as early as 3 days and reach a maximum on day 7 of the culture. Occasionally, cells with a ploidy greater than 16N are detected but neither reaches significant level. This absence of accumulation of polyploidy cells seems to be inherent to the in vitro CD34+ differentiation system, which nevertheless generate within 2 weeks significant numbers of functional human platelets.31 Indeed, after only 3 days, BMP4 and TPO increase from 3- to 6-fold, respectively, compared with nontreated cells and reached, respectively, up to 294 or 196 × 106 platelets/106 CD34+ after 18 days of culture. Platelet production follows the same kinetic in the presence of both cytokines, but TPO seems the most efficient (Table 1). Platelet factor-4 (PF4), a secreted regulator of the late stages of MK, participates in the negative feedback control of megakaryopoieisis and is produced throughout human in vitro MK differentiation32 (Figure S1C). First, we analyzed by RT-PCR the simultaneous expression of FOG-2 and PF4. After 3 days of culture with TPO and BMP4 alone or in combination, the expression of both genes increased in treated cells (Figure 2A), which probably reflects the increasing number of CD41+ cells in the culture (Figure S2B). We further monitored the expression of PF4 protein by Western blot analysis in total cell lysates (Figure 2B) and cell culture supernatants (Figure 2C). After 7 days of treatment with TPO or BMP4, PF4 was induced simultaneously with CD42a (Figure 2B), which is in correlation with results of RT-PCR (Figure 2A) and flow cytometric (Figure 1B) analysis. Finally, we observed in the supernatant at day 10 the expected TPO-induced secretion of PF4, which accumulated with time (day 18; Figure 2C); a similar secretion was seen in the presence of BMP4. With activin A or BMP2, no significant and reproducible induction of PF4 at any level, or elevation of CD42a and FOG-2 above baseline levels were detected (Figure 2).
Effect of BMP4 and TPO on cell ploidization and platelet production from CD34+ cells
| . | Day 3 . | Day 7 . | Day 10 . | Day 18 . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None . | TPO . | BMP4 . | None . | TPO . | BMP4 . | None . | TPO . | BMP4 . | None . | TPO . | BMP4 . | ||
| Ploidy | |||||||||||||
| Sub G1 | 15 ± 11 | 1.5 ± 1 | 2 ± 2 | 8 ± 4 | 5 ± 4 | 5 ± 3 | 11 ± 4 | 5 ± 2 | 5 ± 3 | 20 ± 10 | 9 ± 7 | 10 ± 5 | |
| 2N | 74 ± 7 | 77 ± 8 | 74 ± 9 | 79 ± 4 | 69 ± 4 | 68 ± 3 | 71 ± 4 | 66 ± 4 | 65 ± 3 | 62 ± 7 | 66 ± 7 | 62 ± 6 | |
| 4N | 8.5 ± 9 | 19 ± 8 | 22 ± 8 | 11 ± 3 | 21 ± 3 | 22 ± 4 | 14 ± 3 | 22 ± 2 | 22 ± 3 | 13 ± 3 | 19 ± 2 | 19 ± 2 | |
| 8N | 2 ± 1 | 2 ± 1 | 1.5 ± 0.5 | 2 ± 0.3 | 4.5 ± 1 | 4 ± 1 | 3 ± 1 | 5 ± 1 | 6 ± 1 | 3 ± 1 | 4 ± 1 | 5 ± 1 | |
| > 16N | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 1 ± 0.1 | 1 ± 0.5 | 2 ± 0.5 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 4 ± 2 | |
| n | 3 | 3 | 3 | 9 | 9 | 9 | 8 | 8 | 8 | 5 | 5 | 5 | |
| Platelet ×106/106 initial CD34+ | 10 ± 2.5 | 61 ± 25 | 31 ± 8 | 39 ± 22 | 64 ± 26 | 55 ± 24 | 53 ± 37 | 186 ± 56 | 135 ± 58 | 96 ± 36 | 294 ± 160 | 196 ± 30 | |
| . | Day 3 . | Day 7 . | Day 10 . | Day 18 . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None . | TPO . | BMP4 . | None . | TPO . | BMP4 . | None . | TPO . | BMP4 . | None . | TPO . | BMP4 . | ||
| Ploidy | |||||||||||||
| Sub G1 | 15 ± 11 | 1.5 ± 1 | 2 ± 2 | 8 ± 4 | 5 ± 4 | 5 ± 3 | 11 ± 4 | 5 ± 2 | 5 ± 3 | 20 ± 10 | 9 ± 7 | 10 ± 5 | |
| 2N | 74 ± 7 | 77 ± 8 | 74 ± 9 | 79 ± 4 | 69 ± 4 | 68 ± 3 | 71 ± 4 | 66 ± 4 | 65 ± 3 | 62 ± 7 | 66 ± 7 | 62 ± 6 | |
| 4N | 8.5 ± 9 | 19 ± 8 | 22 ± 8 | 11 ± 3 | 21 ± 3 | 22 ± 4 | 14 ± 3 | 22 ± 2 | 22 ± 3 | 13 ± 3 | 19 ± 2 | 19 ± 2 | |
| 8N | 2 ± 1 | 2 ± 1 | 1.5 ± 0.5 | 2 ± 0.3 | 4.5 ± 1 | 4 ± 1 | 3 ± 1 | 5 ± 1 | 6 ± 1 | 3 ± 1 | 4 ± 1 | 5 ± 1 | |
| > 16N | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 1 ± 0.1 | 1 ± 0.5 | 2 ± 0.5 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 4 ± 2 | |
| n | 3 | 3 | 3 | 9 | 9 | 9 | 8 | 8 | 8 | 5 | 5 | 5 | |
| Platelet ×106/106 initial CD34+ | 10 ± 2.5 | 61 ± 25 | 31 ± 8 | 39 ± 22 | 64 ± 26 | 55 ± 24 | 53 ± 37 | 186 ± 56 | 135 ± 58 | 96 ± 36 | 294 ± 160 | 196 ± 30 | |
Isolated CD34+ cells were incubated in the 4GF serum-free medium in the presence or not of 100 ng/mL of BMP4 or TPO. After 3, 7, 10, or 18 days, cells were counted and assessed for ploidization by flow cytometry using a Facscalibur cell analyzer (BD Biosciences). Results are expressed as mean cells in each ploidy class, and the number of experiments for each time point is indicated as n experiments. The numbers of platelets are expressed as the total number of platelets ×106 per 106 initial CD34+ and represent the mean of 3 independent experiments.
Effects of TPO, activin A, BMP2, and BMP4 on markers of late stages of MK maturation. CD34+ cells were incubated for 3 to 18 days in 4GF serum-free medium in the presence or not of 50 ng/mL TPO, activin A (ACT), BMP2, or BMP4 as indicated. (A) After 3 days, we assayed the expression of PF4 and FOG-2 at the RNA level by RT-PCR analysis, as described in “RNA isolation and analysis.” In all conditions, we found a similar level of expression of the housekeeping gene GAPDH, which allowed us to compare levels of expression of PF4 and FOG-2 between the different treatments. (B) After 7 days of culture, total cell lysates were quantified, and 10 μg total proteins was separated by SDS-PAGE. Immunodetection was performed as described in “Western blot analysis” using monoclonal mouse anti-PF4 and anti-CD42a antibodies, or antiactin used as a loading control. (C) Finally, at the latest stages of in vitro MK differentiation and using 30 μL cell culture supernatant, secreted PF4 was detected after 10 and 18 days of culture. Equal aliquots of the culture supernatant were separated by SDS-PAGE. Immunodetection was performed as described in “Western blot analysis” using monoclonal mouse anti-PF4 antibody.
Effects of TPO, activin A, BMP2, and BMP4 on markers of late stages of MK maturation. CD34+ cells were incubated for 3 to 18 days in 4GF serum-free medium in the presence or not of 50 ng/mL TPO, activin A (ACT), BMP2, or BMP4 as indicated. (A) After 3 days, we assayed the expression of PF4 and FOG-2 at the RNA level by RT-PCR analysis, as described in “RNA isolation and analysis.” In all conditions, we found a similar level of expression of the housekeeping gene GAPDH, which allowed us to compare levels of expression of PF4 and FOG-2 between the different treatments. (B) After 7 days of culture, total cell lysates were quantified, and 10 μg total proteins was separated by SDS-PAGE. Immunodetection was performed as described in “Western blot analysis” using monoclonal mouse anti-PF4 and anti-CD42a antibodies, or antiactin used as a loading control. (C) Finally, at the latest stages of in vitro MK differentiation and using 30 μL cell culture supernatant, secreted PF4 was detected after 10 and 18 days of culture. Equal aliquots of the culture supernatant were separated by SDS-PAGE. Immunodetection was performed as described in “Western blot analysis” using monoclonal mouse anti-PF4 antibody.
In summary, our molecular, phenotypic, and functional analysis showed for the first time that, contrary to activin A and BMP2, BMP4 alone significantly and specifically regulates human megakaryopoiesis, and this effect is maintained up to late stages. However, BMP4 and TPO effects do not appear to be additive.
TPO and BMP4 use the same signaling pathways to regulate human MK
To understand how TPO and BMP4 participate to the regulation of human MK, we compared different signaling pathways involved in human MK regulation, using specific biochemical inhibitors (AG490 for JAK2, PD098059 for ERK1-2, LY294002 for PI3K and rapamycin for mTor).26,28 CD34+ cells were incubated for 3 to 7 days in the presence of TPO or BMP4 used alone or with the indicated inhibitor. Results presented in Figure 3A reveal that if AG490, Ly294002, and rapamycin decreased cell proliferation, only Ly294002 significantly altered the number of viable CD34+ cells treated with either TPO (62% vs 82% with TPO alone) or BMP4 (57% vs 76% with BMP4 alone). This indicates that only the PI3K inhibitor Ly294002 seemed to induce apoptosis (Figure 3A), as already reported for TPO.26,33 This inhibitor also decreased by half the effects of TPO or BMP4 on CFU-MK (Figure 3B) but had no significant effect on CD41 expression (Figure 3C), indicating that MK progenitors are more dependent on PI3K signaling to survive than differentiated cells, as reported for TPO.26 Interestingly, AG490 and rapamycin efficiently inhibited TPO or BMP4 effects on all parameters assayed (Figure 3B,C) without affecting cell viability (Figure 3A). Consistent with previous observations that a regular addition of the MAPK inhibitor is necessary to visualize its effect,26 we did not observe any significant effect of PD98059 compared with other inhibitors. Altogether, these data underline that TPO and BMP4 use the JAK and mTor signaling pathways to regulate human megakaryopoiesis.
Effect of signaling pathway inhibitors on BMP4- and TPO-induced MK differentiation. Purified CD34+ cells were incubated in 4GF serum-free medium in the presence or not of TPO (left panels) or BMP4 (right panels; 50 ng/mL) with or without specific inhibitors of the following signaling pathways: JAK/Stat (AG490, 30 μM), MAPK (PD98059, 2 μM), PI3K (LY294002, 10 μM), and mTOR (rapamycin, 100 nM). (A) After 3 days, cells were harvested and the number of viable cell was evaluated by trypan blue counting. Data represent the percentage of viable cells as a result of the following ratio: (viable cell number/total cell count) × 100. The fold proliferation represents the ratio between the number of viable cells at day 3 and input cells. (B) Cells were placed in functional progenitor assays to determine CFC-MK numbers after 3 days of treatment. Results are expressed as numbers of colonies per 1000 seeded cells. Results (panels A,B) represent the mean plus or minus SEM of 9 experiments. (C) Phenotypic analysis of the early MK specific marker CD41 was performed after 7 days of treatment by flow cytometric analysis using a Facscalibur cell analyzer (BD Biosciences) and gating on viable cells. Results are presented as percentages of positive viable cells plus or minus SEM of 6 experiments. * indicates statistically significant difference from those in nontreated cells (P < .05).
Effect of signaling pathway inhibitors on BMP4- and TPO-induced MK differentiation. Purified CD34+ cells were incubated in 4GF serum-free medium in the presence or not of TPO (left panels) or BMP4 (right panels; 50 ng/mL) with or without specific inhibitors of the following signaling pathways: JAK/Stat (AG490, 30 μM), MAPK (PD98059, 2 μM), PI3K (LY294002, 10 μM), and mTOR (rapamycin, 100 nM). (A) After 3 days, cells were harvested and the number of viable cell was evaluated by trypan blue counting. Data represent the percentage of viable cells as a result of the following ratio: (viable cell number/total cell count) × 100. The fold proliferation represents the ratio between the number of viable cells at day 3 and input cells. (B) Cells were placed in functional progenitor assays to determine CFC-MK numbers after 3 days of treatment. Results are expressed as numbers of colonies per 1000 seeded cells. Results (panels A,B) represent the mean plus or minus SEM of 9 experiments. (C) Phenotypic analysis of the early MK specific marker CD41 was performed after 7 days of treatment by flow cytometric analysis using a Facscalibur cell analyzer (BD Biosciences) and gating on viable cells. Results are presented as percentages of positive viable cells plus or minus SEM of 6 experiments. * indicates statistically significant difference from those in nontreated cells (P < .05).
BMP4 is involved in TPO-induced MK regulation
To go one step further in understanding the relationship between TPO and BMP4, we blocked TPO or BMP4 signaling pathways by adding anti–TPO-R or anti-BMP4 blocking antibodies to CD34+ cells incubated in the presence or not of TPO or BMP4 (Figure 4A). The CFU-MK content was assayed after 3 days of culture and CD41 expression after 7 days. As expected, results validated the efficiency of anti–TPO-R antibodies to inhibit TPO-induced CFU-MK and CD41 (Figure 4A left panel) compared with the isotype control goat IgG. Similarly, anti-BMP4 antibodies significantly blocked the effect of BMP4 on CFU-MK and CD41 expression (Figure 4A right panel) compared with the isotype control IgG2b. Interestingly, anti-BMP4 antibodies strongly inhibited TPO-induced CFU-MK differentiation and CD41 expression (Figure 4A left panel). Inversely, anti-TPO-R antibodies did not block the effects of BMP4 on CFU-MK or CD41 (Figure 4A right panel). These results suggest that TPO uses the BMP4 pathway to regulate human MK, whereas the effects of BMP4 do not appear to depend on TPO signaling.
Inhibition of TPO effects on MK differentiation by extracellular inhibitors of the BMP pathway. Purified CD34+ cells were incubated 3 or 7 days in 4GF serum-free medium in the presence or not (−) of 100 ng/mL of TPO or BMP4 alone, or with specific exogenous inhibitors. (A) Cells were incubated with TPO or BMP4 alone (left and right panels, respectively) and in the presence of blocking goat anti–TPO-R antibody and its isotype control goat IgG (20 μg/mL), or blocking monoclonal mouse anti-BMP4 antibody and its isotype control mouse IgG2b (1 μg/mL). After 3 days of culture, cells were assayed for their CFU-MK content (top panel). Results, expressed as numbers of CFU-MK/1000 seeded cells, represent the mean value plus or minus SEM of 10 experiments. After 7 days, cells were harvested and flow cytometry was used to determine the proportion of CD41+ cells in viable cells (gated; bottom panel). Data represent the mean percentage of positive viable cells plus or minus SEM of 9 experiments. The P value of statistical differences between cells treated by simultaneous addition of TPO or BMP4 with isotype control antibodies or with specific blocking antibodies is directly mentioned in the figure. (B) Cells were incubated in the presence of 50 ng/mL of follistatin (Foll) or follistatin-related gene (FLRG). After 3 days of culture, cells were assayed for their CFU-MK content (left panel) and after 7 days for the percentage of CD41+ viable cells (gated; right panel). Results, expressed as numbers of CFU-MK/1000 seeded cells or percentage of positive viable cells, represent the mean value plus or minus SEM of 5 experiments. (C) After 7 days, cells were harvested and counted, and platelets and supernatant were collected for PF4 quantification. Results are expressed as the total number of platelets × 106 per 106 initial CD34+ and represent the mean of 4 independent experiments. The proportion of CD42b+ cells in viable cells (gated) was determined by flow cytometry. The total number of CD42b+ cells was calculated and reported to the initial number of CD34+ cells. Data represent the mean number of positive CD42b cells/106CD34+ plus or minus SEM of 6 experiments. The supernatant was cleared by 10 minutes of centrifugation at 3000g, then placed in 96-well plates for ELISA quantification of PF4 as described in “BMP4 and PF4 quantification.” Results expressed in pg/mL of PF4 represent the mean value plus or minus SEM of 3 experiments. * indicates statistically significant difference from those in nontreated cells (P < .05).
Inhibition of TPO effects on MK differentiation by extracellular inhibitors of the BMP pathway. Purified CD34+ cells were incubated 3 or 7 days in 4GF serum-free medium in the presence or not (−) of 100 ng/mL of TPO or BMP4 alone, or with specific exogenous inhibitors. (A) Cells were incubated with TPO or BMP4 alone (left and right panels, respectively) and in the presence of blocking goat anti–TPO-R antibody and its isotype control goat IgG (20 μg/mL), or blocking monoclonal mouse anti-BMP4 antibody and its isotype control mouse IgG2b (1 μg/mL). After 3 days of culture, cells were assayed for their CFU-MK content (top panel). Results, expressed as numbers of CFU-MK/1000 seeded cells, represent the mean value plus or minus SEM of 10 experiments. After 7 days, cells were harvested and flow cytometry was used to determine the proportion of CD41+ cells in viable cells (gated; bottom panel). Data represent the mean percentage of positive viable cells plus or minus SEM of 9 experiments. The P value of statistical differences between cells treated by simultaneous addition of TPO or BMP4 with isotype control antibodies or with specific blocking antibodies is directly mentioned in the figure. (B) Cells were incubated in the presence of 50 ng/mL of follistatin (Foll) or follistatin-related gene (FLRG). After 3 days of culture, cells were assayed for their CFU-MK content (left panel) and after 7 days for the percentage of CD41+ viable cells (gated; right panel). Results, expressed as numbers of CFU-MK/1000 seeded cells or percentage of positive viable cells, represent the mean value plus or minus SEM of 5 experiments. (C) After 7 days, cells were harvested and counted, and platelets and supernatant were collected for PF4 quantification. Results are expressed as the total number of platelets × 106 per 106 initial CD34+ and represent the mean of 4 independent experiments. The proportion of CD42b+ cells in viable cells (gated) was determined by flow cytometry. The total number of CD42b+ cells was calculated and reported to the initial number of CD34+ cells. Data represent the mean number of positive CD42b cells/106CD34+ plus or minus SEM of 6 experiments. The supernatant was cleared by 10 minutes of centrifugation at 3000g, then placed in 96-well plates for ELISA quantification of PF4 as described in “BMP4 and PF4 quantification.” Results expressed in pg/mL of PF4 represent the mean value plus or minus SEM of 3 experiments. * indicates statistically significant difference from those in nontreated cells (P < .05).
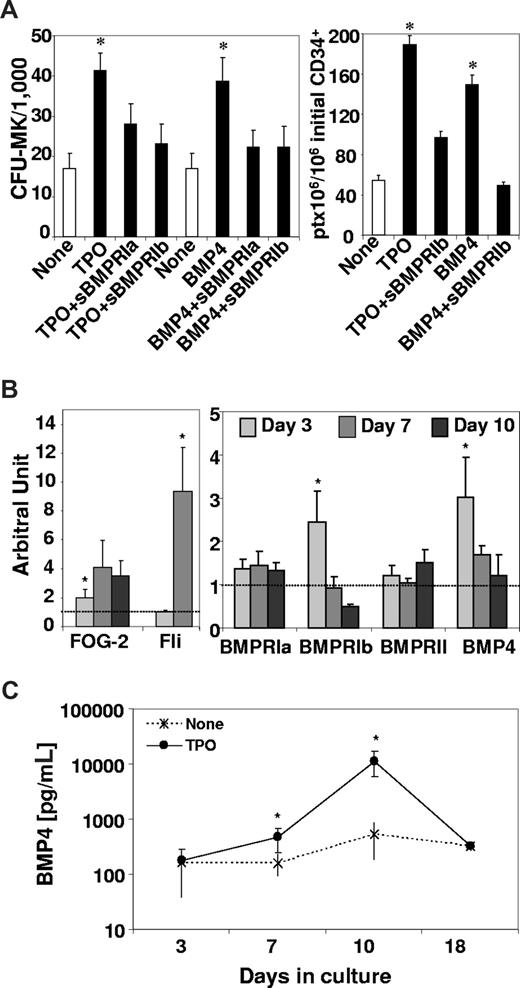
To confirm these findings, we used 2 known specific physiologic inhibitors of BMP4, FLRG, and follistatin3,9 and evaluated their effects on TPO-induced CFU-MK and CD41 expression. Results presented in Figure 4B show that, as expected, FLRG or follistatin blocked BMP4 effects on the growth of CFU-MK or CD41. Moreover, FLRG and follistatin decreased the effects of TPO on CFU-MK or CD41 (Figure 4B). Finally, we analyzed late MK steps by evaluating the total number of CD42+ cells (which reliably represent mature MK cells, as reported32 ), the total platelets produced and the level of PF4 in culture supernantant after 7 days with the same amount of CD34+ cells in the presence or not of various inhibitors (Figure 4C). Results show that both TPO and BMP4 allowed very efficient production of mature MK cells approximately 0.6 × 106 CD42b+/106 CD34+. This effect was efficiently blocked by different BMP inhibitors (follistatin, FLRG, or blocking antibodies). Similarly TPO- and BMP4-induced platelet production and PF4 synthesis was decreased by follistatin (Figure 4C). We inhibited the BMP signaling pathway as described13 by adding soluble BMP receptors type Ia (sBMPRIa) and Ib (sBMPRIb) to TPO- or BMP4-treated CD34+ cells (Figure 5A). After 3 days, the MK progenitor content was assayed, and both soluble BMP receptors were shown to inhibit the CFU-MK growth-mediated by TPO or BMP4 compared with cytokines alone. Similarly, after 7 days, TPO- and BMP4-induced platelet production was efficiently blocked by soluble BMPRIb. Altogether, these data confirmed the results obtained with either blocking antibodies or natural inhibitors (Figure 4), strongly indicating that TPO uses the BMP4 signaling pathway during all stages of MK differentiation.
TPO induces the BMP4 autocrine loop in MK progenitors. Purified CD34+ cells (6 × 105/mL) were cultured in 4GF serum-free medium supplemented with TPO or BMP4 (100 ng/mL). (A) Cells were incubated in the presence of type Ia (sBMPR-Ia, 1 μg/mL) and type Ib (sBMPR-Ib, 4 μg/mL) soluble BMP receptors to inhibit the BMP signaling pathway as described.13 After 3 days of culture, wells were assayed for their CFU-MK and after 7 days for their platelet content. Results represent the mean value plus or minus SEM of, respectively, 6 and 4 experiments. (B) RT-PCRq expression data are expressed as a mean ratio of the gene of interest to 2 control genes, TBP and COF. Results are expressed as a fold increase calculated from the ratio of TPO-treated cells to nontreated cells. Therefore, the 1 value represented on the graph matches the baseline level of the gene in nontreated cells. Results represent the mean value plus or minus SEM of 5 independent experiments. (C) After 7 days of culture, the supernatant was harvested and cleared by 10 minutes of centrifugation at 3000g, then placed in 96-well plates for ELISA quantification of BMP4 as described in “BMP4 and PF4 quantification.” Results expressed in pg/mL of BMP4 represent the mean value plus or minus SEM of 4 to 9 experiments. * indicates statistically significant difference from those in nontreated cells (P < .05).
TPO induces the BMP4 autocrine loop in MK progenitors. Purified CD34+ cells (6 × 105/mL) were cultured in 4GF serum-free medium supplemented with TPO or BMP4 (100 ng/mL). (A) Cells were incubated in the presence of type Ia (sBMPR-Ia, 1 μg/mL) and type Ib (sBMPR-Ib, 4 μg/mL) soluble BMP receptors to inhibit the BMP signaling pathway as described.13 After 3 days of culture, wells were assayed for their CFU-MK and after 7 days for their platelet content. Results represent the mean value plus or minus SEM of, respectively, 6 and 4 experiments. (B) RT-PCRq expression data are expressed as a mean ratio of the gene of interest to 2 control genes, TBP and COF. Results are expressed as a fold increase calculated from the ratio of TPO-treated cells to nontreated cells. Therefore, the 1 value represented on the graph matches the baseline level of the gene in nontreated cells. Results represent the mean value plus or minus SEM of 5 independent experiments. (C) After 7 days of culture, the supernatant was harvested and cleared by 10 minutes of centrifugation at 3000g, then placed in 96-well plates for ELISA quantification of BMP4 as described in “BMP4 and PF4 quantification.” Results expressed in pg/mL of BMP4 represent the mean value plus or minus SEM of 4 to 9 experiments. * indicates statistically significant difference from those in nontreated cells (P < .05).
TPO induces a BMP4 autocrine loop in human MK progenitors
Finally, to evaluate the impact of TPO on the extracellular elements required to initiate the BMP4 pathway, we analyzed its effects on BMP4 and BMP receptor expression. After 3 to 10 days of exposure to TPO, we monitored by RT-PCRq the expression of BMP4, BMP type I (BMPRIa and BMPRIb) and type II (BMPRII) receptors, both types being required for signal transduction.19 As a control for MK differentiation, we also quantified FOG-2 and Fli1 in the same samples. Results confirmed the early (Figure 2A) and transient induction of FOG-2, which correlated with an increased expression of the MK-specific transcription factor Fli1,24,25 and interestingly showed that the TPO-induced expression of FOG-2 precedes the one of Fli1 (Figure 5B). Simultaneously, we assayed the expression of BMP4 and BMPR in the same samples. We observed a rapid and significant increase (day 3) of BMP4 and BMPRIb followed by a decrease of their expression (Figure 5B). A modest effect was observed on types Ia and II receptors expression, which however seemed to be maintained over time. The early increase of BMP4 RNA expression is followed by protein synthesis as shown by quantification of soluble BMP4 in culture supernatant of TPO-treated cells. Untreated CD34+ cells placed in the MK in vitro differentiation system synthesized their own BMP4 (154 pg/mL; Figure 5C) and TPO addition significantly increased this self-production by more than 3- to 21-fold after, respectively, 7 to 10 days. Altogether, these results indicated that TPO activates BMP4 signaling in MK progenitors by inducing the synthesis of BMP4 and of its receptors.
Discussion
We investigated the role of activin A, BMP2, and BMP4 during human megakaryopoiesis using an in vitro differentiation system that enabled to reproduce the major steps of MK maturation and generate enough cells to perform the required analysis. We report here that activin A and BMP2 do not modulate MK differentiation, as we could not show any effect on either early or late maturation steps. Conversely, we have previously shown that, in the absence of EPO, activin A or BMP2 alone induces the commitment of primary human precursors toward the erythroid lineage.3 However, it becomes clear from the literature that BMP4 can also regulate human and murine erythropoiesis but requires the presence of EPO.8,34 Our previous and present data suggest that activin A and BMP2 are involved in the regulation of erythropoiesis,3 but not in megakaryopoiesis, and underline that in humans, conversely to other animal models,11,22 BMP2 and BMP4 do not share a redundant biologic function in the regulation of hematopoiesis.3
We have shown that BMP4 cooperates with SCF to modulate the primitive hematopoietic stem cell compartment in the absence of any other cytokine. This is in agreement with recent observations in mice where BMP4 efficiently cooperates with SCF and EPO to regulate erythropoiesis34 and with increasing evidence that BMP4 stands out from other members of the TGF-β family in terms of stem cell regulation.13,15,35 Our data document here for the first time the modulation by BMP4 alone of several parameters involved in the differentiation toward the human MK lineage, such as a significant increase of CFU-MK sustained throughout time, inversely to TPO. This is in line with the ability of BMP4 to affect very primitive compartments12,35 and indicated here by its effect on LTC-IC.
We have analyzed the expression pattern of FOG-1 and FOG-2 during in vitro human megakaryopoiesis, and shown that, although both human FOG-1 and FOG-2 are rapidly induced, FOG-2 appears to be more expressed in progenitors and early MK cells. This is in line with observations in Xenopus and Drosophila36,37 that FOG-2 expression inhibits erythropoiesis and is probably required to allow engagement and progression toward MK differentiation. Indeed, the in vitro transient expression of FOG-2 precedes that of the MK-specific transcription factor Fli1.24,25 Our data thus highlight the potential role of FOG-2 in early steps of human MK differentiation.
During the MK differentiation process, we have demonstrated that, of the TGF-β family, only BMP4 has the same capacity as TPO to induce early and late MK markers, and similar terminal differentiation properties, such as polyploidization, secretion of PF4, and platelet production. The pivotal role of TPO is supported by the abrogation of either TPO or its receptor (c-mpl) in mice displaying almost 85% reduction in marrow MK numbers and circulating platelets.38 However, the presence in these mice of spare normal MK and platelets39 and the correction of thrombocytosis by 5-fluorouracil treatment40 suggest that TPO is important but not essential for megakaryopoiesis. Together with other observations,41 these findings indicate that cytokines or microenvironment signals can elicit a TPO-independent megakaryopoiesis. Indeed, cytokines that signal through gp130 proteins (IL-6, IL-11) exhibit a significant megakaryopoietic potential, which is however unable to compensate for TPO deficiency.42 In TPO/mpl deficient mice, it is obvious that interactions of progenitors with the bone marrow vascular niche induced by SDF-1 and FGF-4 support the maturation of MK.41 This major TPO-independent role played by the marrow microenvironment in the regulation of murine thrombopoiesis is also possible in humans. Here, we have demonstrated that BMP4, an element of a key signaling pathway involved in the regulation of the hematopoietic “niche,”7 which is mainly produced by the bone marrow stroma,29 localized in human megakaryocytes and platelets43 and autologously produced by MK progenitors, efficiently regulates all stages of human megakaryopoiesis, from maintenance of primitive uncommitted progenitors to late stages of MK differentiation. This provides evidence for a significant role of the BMP signaling pathway in processes controlling human MK regulation. A similar mechanism probably occurs in vivo, which requires further investigation and validation. In the past, the discovery of platelet growth factors raised expectations that an effective method for abrogating thrombocytopenia would become available in the clinic. Although several cytokines have been evaluated for their ability to reduce thrombocytopenia in a variety of clinical scenarios, their development has been abandoned because of their limited effectiveness or excessive toxicity. Clinical results with TPO were more promising, but its repeated use has resulted in the development of neutralizing antibodies, which has precluded its further clinical development. A growing number of new nonimmunogenic peptide and nonpeptide TPO agonists have recently been tested in clinical trials; they provide effective treatment with acceptable toxicity, but additional clinical evaluation is required before their approval for clinical use.44 In this context, the use of BMP4 might be worth considering as BMP proteins are already in clinical use for bone healing.45
We have investigated the possible relationship between BMP4 and TPO using specific biochemical inhibitors of the signaling pathways relevant for MK regulation.25 Our data suggest that the JAK/STAT and mTor signaling pathways are involved in the regulation of MK maturation by BMP4, and this has also been confirmed for TPO.26,28 This is consistent with the involvement of mTor and STAT3 signaling pathway in BMP4-mediated cell fate in the central nervous system.18 Our results then indicate that BMP4 and TPO use similar signaling pathways to regulate human MK differentiation. Using specific extracellular inhibitors of TPO or BMP4, we have shown that, whereas either inhibitor of the BMP4 signaling pathway efficiently inhibited the effects of TPO, anti-TPO-R antibodies were not able to block the effects of BMP4 on MK differentiation. Moreover, TPO induced BMP4 synthesis and BMP receptor expression in MK progenitors. Altogether, these results show that, whereas TPO uses the BMP4 signaling pathway to regulate human MK, the reverse does not seem to be true.
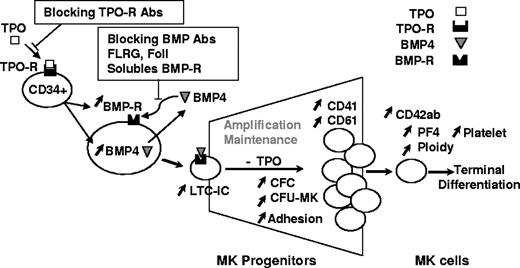
In conclusion, our results provide new insights on the role of TPO and BMP4 in the regulation of human primary MK cells and suggest for the first time that BMP4 signaling is involved in TPO activity (proposed model in Figure 6). Interestingly, at the clinical level, our work also opens new perspectives for the treatment of diseases that combine bone abnormalities and a deregulation of megakaryopoiesis. Indeed, our results, together with the fact that BMP proteins are produced by MK cells and platelets,43 suggest that a deregulation in the BMP4 signaling pathway might be involved in such diseases, as suggested in multiple myeloma.46 Subsequently, it would be interesting to analyze the integrity of the BMP4 pathway, both in the bone marrow structure and in hematopoietic megakaryocytes. It might be particularly relevant to explore it in the context of chronic myeloid leukemia, which is characterized by profound alterations of the bone marrow structure and frequent deregulations of megakaryopoiesis, independently from TPO signaling pathways.47
Proposed model for TPO and BMP4 effects during human megakaryopoiesis.
Proposed model for TPO and BMP4 effects during human megakaryopoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Bordet (Hospital Edouard Herriot, Lyon), Dr Louache (U790, IGR, Villejuif), and Dr Coulombel (U602, Hôpital Paul Brousse, Villejuif) for helpful discussions, and Elodie Bachelard, Stéphane Joly, Gaëtan Pochon, and the cell sorting facilities of Centre Leon Bérard and IFR128 for their excellent technical assistance.
This study was supported in part by research funding from Inserm (V.M.-S., A.P.), the Association pour la Recherche contre le Cancer (R.R.), and the Ligue Nationale contre le Cancer (Rhône, Drôme; R.R.).
Authorship
Contribution: S.J. and B.K. performed the experiments; F.E.N. provided the primary cells, analyzed the data, and contributed to manuscript writing; C.D. and R.R. contributed to research design and proofread the manuscript; A.P. contributed to discussions during the project and contributed to manuscript writing; and V.M.-S. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Véronique Maguer-Satta, Inserm U590, Centre Léon Bérard, 28 Rue Laennec, 69373 Lyon Cedex 08, France; e-mail: maguer@lyon.fnclcc.fr.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal