Abstract
Phenotypically and functionally distinct progenitors and developmental pathways have been proposed to exist for fetally derived B-1 and conventional B-2 cells. Although IL-7 appears to be the primary cytokine regulator of fetal and adult B lymphopoiesis in mice, considerable fetal B lymphopoiesis and postnatal B cells are sustained in the absence of IL-7; in humans, B-cell generation is suggested to be largely IL-7–independent, as severe combined immune-deficient patients with IL-7 deficiency appear to have normal B-cell numbers. However, the role of other cytokines in IL-7–independent B lymphopoiesis remains to be established. Although thymic stromal lymphopoietin (TSLP) has been proposed to be the main factor driving IL-7–independent B lymphopoiesis and to distinguish fetal from adult B-cell progenitor development in mice, recent studies failed to support a primary role of TSLP in IL-7–independent fetal B-cell development. However, the role of TSLP in IL-7–independent adult B lymphopoiesis and in particular in regulation of B-1 cells remains to be established. Here we demonstrate that, rather than TSLP, IL-7 and FLT3 ligand are combined responsible for all B-cell generation in mice, including recently identified B-1–specified cell progenitors. Thus, the same IL-7– and FLT3 ligand–mediated signal-ing regulates alternative pathways of fetal and adult B-1 and B-2 lymphopoiesis.
Introduction
Human immunodeficiencies have uncovered the key role of cytokine signaling pathways in T lymphocyte development, facilitating development of cell replacement and gene therapies.1,2 In contrast, little is known about the cytokine pathways that might be perturbed in immunodeficiencies with a B-cell phenotype
In mice, fetal and adult B-cell development has been suggested to be regulated by overlapping yet largely distinct cytokine ligands and receptors.3-9 Specifically, whereas conventional B-2 B lymphopoiesis in the adult bone marrow (BM) is lost in the absence of only interleukin-7 (IL-7), fetal and early postnatal B lymphopoiesis, in particular B-1 B-cell genesis, seems to be largely driven by IL-7–independent mechanisms.10-12 Importantly, such IL-7–independent B lymphopoiesis is sufficient to sustain a pool of peripheral B cells and normal immunoglobulin levels through life.3,13
Notably, also human B-cell development appears to be largely or entirely IL-7–independent, as X-linked severe combined immunodeficiency caused by a loss of function mutation in the IL-2 receptor gamma chain (IL-2Rg), as well as IL-7 receptor alpha chain (IL-7Rα) deficiency, are accompanied by severely defective T lymphopoiesis but apparently normal B-cell numbers.14-16 Thus, it could have considerable clinical implications to establish which alternative cytokines might be involved in regulating IL-7–independent B lymphopoiesis.
Several previous lines of evidence strongly implicated thymic stromal lymphopoietin (TSLP) as a primary regulator of IL-7–independent fetal B lymphopoiesis.8,9 TSLP shares with IL-7 the IL-7Rα as a ligand-binding receptor subunit. However, whereas IL-7–dependent signaling in addition depends on association with the IL-2Rg, shared with multiple other lymphokines,14 TSLP-mediated signaling rather acts through an IL-2Rg–like TSLP receptor (TSLPR) unit.17,18 Accordingly, and in contrast to IL-2Rg and IL-7–deficient (Il2rg−/− and Il7−/−) mice, IL-7Rα–deficient (Il7r−/−) mice lack IL-7 as well as TSLP-mediated signaling. One of the major reasons that TSLP has been implicated as an important regulator of IL-7–independent B lymphopoiesis is the 10-fold further reduction in B-cell progenitors and mature B cells observed in adult Il7r−/− mice compared with Il7-deficient mice.8,9 However, few direct comparisons of Il7−/− and Il7r−/− mice have been made and largely performed on mice of mixed genetic backgrounds.11,19,20 In support of a unique role of TSLP in embryonic rather than adult B lymphopoiesis, fetal but not adult conventional pro-B cells have been shown to be TSLP responsive,8,9 and also the residual compartment of B-1 B-cell progenitors in adult BM sustains TSLP responsiveness.6 Whereas a lack of an important role of TSLP alone in adult B lymphopoiesis has been established in TSLPR-deficient (Tpte2−/−) mice,6,21,22 its proposed role in fetal B-cell development was based on comparative studies in postnatal Il7r- and Il7-deficient mice and differences in TSLP responsiveness between fetal and adult B-cell progenitors.6,8,9 Notably, more recent and direct analysis of Tpte2 and Il7 double-deficient embryos failed to confirm an important role of TSLP in IL-7–independent fetal B lymphopoiesis.23 As human IL7R-deficient patients have normal B-cell numbers,1,24 although B lymphopoiesis in these patients has not been investigated in detail and only in infants, this further questions the importance of TSLP in conventional fetal B lymphopoiesis. However, these findings do not rule out an important role of TSLP in other aspects of IL-7–independent B lymphopoiesis, neither in adult B lymphopoiesis, and in particular not in B-1 B lymphopoiesis which has been demonstrated to be largely IL-7–independent.3 On the contrary, the potential key role of TSLP in IL-7–independent B-cell development has been reinforced25 and therefore remains controversial.
Previous findings have raised but not addressed several key questions relating to the proposed role of TSLP and other cytokines in postnatal and fetal IL-7–independent B lymphopoiesis. First, what, if any, is the role of TSLP in postnatal B-2 lymphopoiesis, as implicated by Il7r−/− mice having a more severe B-cell phenotype than Il7−/− mice? Second, as mice double-deficient in Il7 and Tpte2 sustain significant fetal B-cell genesis and postnatal B cells, other identified or novel growth factors might play essential roles in promoting IL-7–independent B lymphopoiesis8,9,21 ; and although TSLP might not play a primary role in IL-7–independent B lymphopoiesis, it could be involved in conjunction with other cytokines. Third, TSLP might as implicated play a unique role in IL-7–independent regulation of distinct B-1 B progenitors.6,25 Importantly, as B-1 B cells can be maintained through peripheral expansion,26 normal levels of B-1 B cells do not rule out a deficiency in B-1 B lymphopoiesis in the fetal liver or BM.
Previous studies of mice double-deficient in FMS-like tyrosine kinase receptor (FLT3 also called FLK227 ) or its ligand (FLT3L) and IL-7Rα (Flt3−/−Il7r−/− and Flt3l−/−Il7r−/− mice) have suggested important roles for IL-7, TSLP, and FLT3L in postnatal B-cell development.8,28 However, the relative importance and interdependence of these cytokines in B-cell development could not be established, as the crossing of Flt3l−/− and Il7r−/− mice precluded dissection of the relative roles of IL-7 and TSLP in FLT3L-indpendent B lymphopoiesis.
Herein, we explored B lymphopoiesis in Tpte2−/− as well as Il7−/−Tpte2−/− mice. In the absence of evidence for any important role of TSLP in IL-7–dependent or –independent B-1 and B-2 B lymphopoiesis, we also investigated whether lack of TSLP function might be involved in the almost complete loss of fetal B-1 and postnatal B-2 B-cell development reported in mice double-deficient in FLT3 and IL-7Rα signaling.8,28 However, whereas TSLPR deficiency failed to have any further effect on postnatal B-cell development in Flt3l−/− or Il7−/− mice, we demonstrate that a combined loss of FLT3L and IL-7 is sufficient to lose all discernable stages of neonatal and adult B lymphopoiesis. For the first time, we also identified FLT3L and IL-7, and not TSLP, as essential regulators of the recently identified B-1–specified B-cell progenitors.6 Thus, we here unequivocally establish that FLT3L is the main cytokine driving IL-7–independent B-1 as well as B-2 B lymphopoiesis.
Methods
Mice
Flt3l−/− mice29 were on a pure C57BL/6 background. Il7r−/− mice20 were from The Jackson Laboratory (Bar Harbor, ME) and had been backcrossed for a total of 10 generations with C57BL/6 mice, as had Il7−/−10 mice. WT C57BL/6 mice from The Jackson Laboratory were used as controls in the Il7r−/− and Il7−/− experiments. Tpte2−/− mice22 were on a mixed 129/SvJ/C57BL/6 background, and WT littermates were used as controls. Double-deficient mice were generated through intercrossing the aforementioned strains except for the mice double-deficient in FLT3L and IL-7Rα as they were obtained by cross breeding of Flt3l−/− (pure C57BL/6) mice with Il7r−/− mice backcrossed 5 generations to C57BL/6 background.28 All experiments were approved by the Ethical Committee at Lund University.
Antibodies
Antibodies used for cell surface staining were as follows: 2.4G2 (CD16/32), RA3-6B2 (B220), RB6-8C5 (Gr1), M1/70 (CD11b, Mac1), 53-7 (CD5), 53-6.7 (CD8α), LY-76 (Ter-119), ID3 (CD19), AA4.1 (AA4.1), S7 (CD43), R6-60.2 (IgM), AL-21 (Ly6c), PK136 (NK1.1), H59-597 (TCRβ), GL3 (TCRγδ) (all from BD Biosciences PharMingen, San Diego, CA), and MB19-1 (CD19) from (eBioscience, San Diego, CA).
Fluorescence-activated cell sorting
B-cell progenitors were defined as pre-pro-B (B220+CD43+AA4.1+CD19−), pro-B (B220+CD43+AA4.1+CD19+), pre-B (B220+CD43−IgM−), and imm-B/mature-B (imm-B, B220+CD43−IgM+). B-1 progenitors were analyzed in perinatal mice at the day of birth and defined as Lineage− (anti-Ly6c, -CD8α, -CD11b, -Gr1, -IgM, -TCRβ, -TCRγδ, -NK1.1, and -Ter119), AA4.1+, CD19+, and B220lo/neg.6 In the case of pre-B and imm-B/mature-B, 7 amino-actinomycin D (Sigma-Aldrich, St Louis, MO) was used to exclude dead cells and for the B-1 B progenitor staining ToPro-1 (Invitrogen, Carlsbad, CA) was used to as a viability dye. Data acquisition was performed on a FACSCalibur (BD Biosciences, San Jose, CA). Data analysis was carried out using FlowJo software (TreeStar, Ashland, OR).
Statistics
All results are expressed as means (SD). The statistical significance between 2 groups of mice with deletion of single versus multiple signaling pathways was determined using a one-tailed Student's t test as removal of additional signaling pathways was hypothesized to result in the same or a more severe B-cell phenotype. Aspin-Welch correction was used in cases of unequal variances between groups.
Results
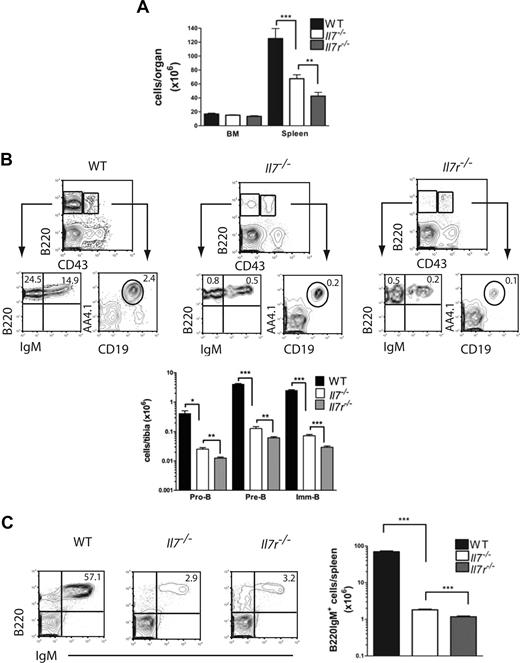
Multiple previous studies implicated a considerably more severe B-cell phenotype in Il7r−/− than Il7−/− mice,3,8,9,11,19,30 indirectly providing evidence for a role of TSLP in IL-7–independent lymphopoiesis. However, the only study that compared B-cell progenitors in the 2 mouse strains side by side and on identical genetic backgrounds did indeed fail to confirm any significant difference in B-cell generation in Il7r−/− and Il7−/− mice.12 In addition, direct comparison of embryonic B-cell development in Il7−/− and Tpte2−/−Il7−/− fetuses revealed only small differences in immature-B (imm-B) cells in fetal liver, and no significant differences in B-cell progenitors.23 In an effort to resolve the apparent discrepancy between previous studies and to potentially reveal a function of TSLP in postnatal B lymphopoiesis in the absence of IL-7, we first investigated the progression from pro-B to imm/mature-B cells in the BM of age-matched Il7−/− and Il7r−/− mice, both backcrossed for more than 10 generations to a C57BL/6 background. Because both IL-7Rα– and IL-7–deficient mice have an age-progressive loss of B-cell progenitors,3,8,31 we chose to study 3-week-old mice in which both mouse strains, although severely reduced in numbers, sustain all stages from pro-B to mature-B cells. Whereas BM cellularity was not significantly different, spleen cellularity was further reduced in Il7r−/− mice compared with Il7−/− mice (1.6-fold; Figure 1A). Further, Il7r−/− mice revealed reductions in frequencies as well as numbers of pro-B, pre-B, and imm/mature-B cells in the BM (Figure 1B), and numbers of mature B cells in the spleen (Figure 1C), compared with Il7−/− mice.
Exacerbated B-cell phenotype in IL-7Rα compared with IL-7 ligand-deficient mice. (A) Mean (SD) tibia and spleen cellularities. (B) Fluorescence-activated cell sorting (FACS) profiles and mean (SD) numbers of pro-B, pre-B, and imm-B cells in BM and (C) mean (SD) numbers of B220IgM+ cells in spleens of 3-week-old WT (n = 4), Il7−/− (n = 8), and Il7r−/− (n = 8) mice (each from 2 litters). Numbers in FACS profiles show the mean percentages of cells in quadrants or gates as indicated (*P < .05, **P < .01, ***P < .001).
Exacerbated B-cell phenotype in IL-7Rα compared with IL-7 ligand-deficient mice. (A) Mean (SD) tibia and spleen cellularities. (B) Fluorescence-activated cell sorting (FACS) profiles and mean (SD) numbers of pro-B, pre-B, and imm-B cells in BM and (C) mean (SD) numbers of B220IgM+ cells in spleens of 3-week-old WT (n = 4), Il7−/− (n = 8), and Il7r−/− (n = 8) mice (each from 2 litters). Numbers in FACS profiles show the mean percentages of cells in quadrants or gates as indicated (*P < .05, **P < .01, ***P < .001).
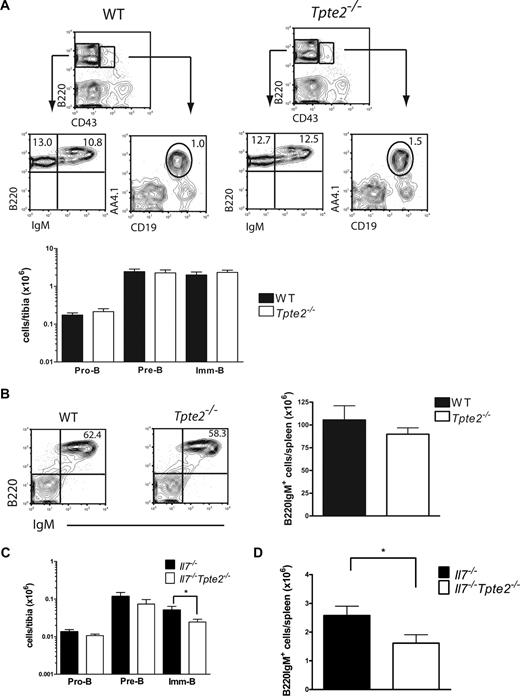
These findings indicate, through an indirect approach, a potential significant, although limited, role of TSLP in adult IL-7–independent B-cell development. However, to more directly investigate the role of TSLP in IL-7–independent postnatalB lymphopoiesis, we next studied B-cell development in Tpte2−/− and Il7−/−Tpte2−/− mice. Previous studies of Tpte2−/− mice failed to establish a role of TSLP alone in adult conventional B-cell lymphopoiesis.21,22 Here, we confirmed and extended these findings by a careful staging of pro-B to imm/mature-B cells30,32 and found no effect of the TSLPR deficiency alone at any B-cell stage in steady-state adult BM (Figure 2A) or spleen (Figure 2B). In mice double-deficient in IL-7 and TSLPR, small (nonsignificant) differences in pro-B- and pre-B-cell numbers as well as a significant reduction in imm-B and splenic B cells were observed compared with IL-7–deficient mice (Figure 2C,D), resembling at least to some degree the further reduction in Il7r−/− mice compared with Il7−/− mice, confirming a significant but very modest role of TSLP in promoting postnatal B lymphopoiesis in the absence of IL-7.
Limited role of TSLP in IL-7–independent BM B lymphopoiesis. (A) FACS profiles (top) and mean (SD) numbers (bottom) of pro-B, pre-B, and imm-B cells in BM of 9- to 11-week-old WT littermate (n = 5) and Tpte2−/− (n = 8) mice (each from 3 litters). (B) FACS profiles and mean (SD) numbers of B220IgM+ cells in spleen of 9- to 11-week-old WT littermate (n = 3) and Tpte2−/− (n = 13) littermate controls. (C) Pro-B, pre-B, and imm-B cells in BM and (D) B220IgM+ cells in spleen of 3-week-old littermate Il7−/− and Il7−/−Tpte2−/− mice (n = 7 and n = 12, 4 litters). Numbers in FACS profiles show the mean percentage of cells within the indicated gates or quadrants of total BM or spleen cells (*P < .05).
Limited role of TSLP in IL-7–independent BM B lymphopoiesis. (A) FACS profiles (top) and mean (SD) numbers (bottom) of pro-B, pre-B, and imm-B cells in BM of 9- to 11-week-old WT littermate (n = 5) and Tpte2−/− (n = 8) mice (each from 3 litters). (B) FACS profiles and mean (SD) numbers of B220IgM+ cells in spleen of 9- to 11-week-old WT littermate (n = 3) and Tpte2−/− (n = 13) littermate controls. (C) Pro-B, pre-B, and imm-B cells in BM and (D) B220IgM+ cells in spleen of 3-week-old littermate Il7−/− and Il7−/−Tpte2−/− mice (n = 7 and n = 12, 4 litters). Numbers in FACS profiles show the mean percentage of cells within the indicated gates or quadrants of total BM or spleen cells (*P < .05).
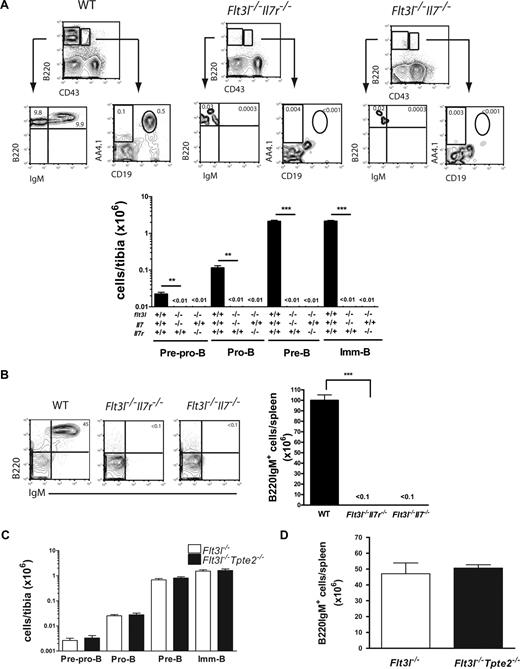
Strikingly, previous studies of Flt3l (or Flt3 receptor) and Il7r double-deficient mice, which lack FLT3L, IL-7, as well as TSLP-mediated signaling, failed to demonstrate detectable numbers of committed B-cell progenitors or mature B cells.8,28 To clarify the relative role of these 3 cytokines in this complete loss of B lineage development, we generated and examined Flt3l−/−Il7−/− as well as Flt3l−/−Tpte2−/− double-deficient mice. Notably, Flt3l−/−Il7−/− as Flt3l−/−Il7r−/− mice were indistinguishable in that they both sustained little or no B-cell progenitors or mature B cells (Figure 3A,B), whereas, in striking contrast, the B-cell phenotype of Flt3l−/−Tpte2−/− mice was indistinguishable from that of Flt3l−/− mice (Figure 3C,D), establishing a primary role of FLT3L, and not TSLP in IL-7–independent B lymphopoiesis.
Critical role of FLT3L but not TSLP in IL-7–independent B lymphopoiesis. (A) FACS profiles and mean (SD) numbers of pre-pro-B, pro-B, pre-B, and imm-B cells in BM of 10- to 11-week-old WT (n = 4), Flt3l−/−Il7r−/− (n = 7-8), and Flt3l−/−Il7−/−(n = 11) mice. (B) FACS profiles and mean (SD) numbers of B220IgM+ cells in spleens of 10- to 11-week-old WT (n = 4), Flt3l−/−Il7r−/− (n = 6), and Flt3l−/−Il7−/−(n = 9) mice. (C) Mean (SD) numbers of pre-pro-B, pro-B, pre-B, and imm-B cells in BM and (D) B220IgM+ cells in spleen of littermate Flt3l−/−(n = 6, 3 litters) and Flt3l−/−Tpte2−/−(n = 6, 3 litters) mice. Numbers in FACS profiles show the mean percentages of cells in quadrants or gates as indicated of total BM and spleen cells (**P < .01, ***P < .001).
Critical role of FLT3L but not TSLP in IL-7–independent B lymphopoiesis. (A) FACS profiles and mean (SD) numbers of pre-pro-B, pro-B, pre-B, and imm-B cells in BM of 10- to 11-week-old WT (n = 4), Flt3l−/−Il7r−/− (n = 7-8), and Flt3l−/−Il7−/−(n = 11) mice. (B) FACS profiles and mean (SD) numbers of B220IgM+ cells in spleens of 10- to 11-week-old WT (n = 4), Flt3l−/−Il7r−/− (n = 6), and Flt3l−/−Il7−/−(n = 9) mice. (C) Mean (SD) numbers of pre-pro-B, pro-B, pre-B, and imm-B cells in BM and (D) B220IgM+ cells in spleen of littermate Flt3l−/−(n = 6, 3 litters) and Flt3l−/−Tpte2−/−(n = 6, 3 litters) mice. Numbers in FACS profiles show the mean percentages of cells in quadrants or gates as indicated of total BM and spleen cells (**P < .01, ***P < .001).
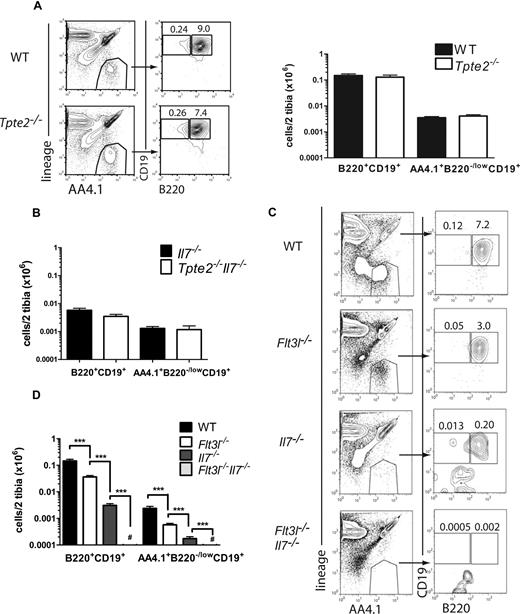
B-1 B cells represent a distinct population of embryonic and early postnatally derived self-replenishing B cells, proposed to be regulated through mechanisms distinct from those of conventional B-2 B cells.7,33,34 Recently, a population of B-1 B progenitors (lin−AA4.1+CD19+B220−/low) was identified in fetal liver, which is sustained at low levels also in postnatal BM.6 These B-1 B progenitors were found to be highly TSLP-responsive; and although present at normal numbers in the BM of adult Tpte2−/− mice,6 a potential important role of TSLP in regulation of B-1 B progenitor has been proposed.25 However, the role of TSLP or other cytokines in regulating IL-7–independent B-1 B progenitors in late fetal development or perinatal BM has not been investigated. Here, B-1 B-cell progenitors were found to be present at normal levels in the BM of newborn Tpte2−/− mice (Figure 4A). Furthermore, IL-7–independent generation of B-1 B-cell progenitors was also not driven by TSLP, as no further reduction was observed in Tpte2−/−Il7−/− mice compared with Il7−/− mice (Figure 4B). In contrast, B-1 B progenitor numbers were reduced 4-fold and 40-fold in Flt3l−/− and Il7−/− mice, respectively, and were undetectable in Flt3l−/−Il7−/− mice (Figure 4C,D), demonstrating the critical importance and interdependence of FLT3L and IL-7 also in regulation of B-1 cell progenitors, and an inability of TSLP to rescue any fetal B-1 or B-2 B-cell development in the absence of FLT3L– and IL-7–mediated signaling.
Absolute requirement of FLT3L but not TSLP in perinatal B-1 lymphopoiesis. (A) FACS profiles and mean (SD) numbers of total lin− AA4.1+B220+CD19+ cells and lin− AA4.1+CD19+B220−/low B-1 progenitors in the BM of 1-day-old WT littermate and Tpte2−/− (n = 8 and n = 6, 2-3 litters) mice. (B) Mean (SD) numbers of total lin−AA4.1+B220+CD19+ cells and B-1 progenitors in 1-day-old BM of littermate Il7−/− (n = 6) and Tpte2−/−Il7−/− (n = 5). (C) FACS profiles and (D) mean (SD) numbers of total lin−AA4.1+B220+CD19+ cells and lin− AA4.1+CD19+B220−/low B-1 progenitors in the BM of 1-day-old WT (n = 16), Flt3l−/− (n = 12), Il7−/− (n = 10), and Flt3l−/−Il7−/− (n = 11) mice (each from at least 2 litters). Numbers in FACS profiles show the percentages of cells within the indicated gates of total BM cells (mean values from all analyzed mice). For panel C, a range of 20 764 to 137 438, 36 500 to 87 584, 22 367 to 74 650, and 16 809 to 143 764 cell events were acquired for the WT, Flt3l−/−, Il7−/−, and Flt3l−/−Il7−/− samples, respectively. # The majority of Flt3l−/−Il7−/− mice had no detectable B-1 progenitors by FACS, and none of the mice had more than 100 cells per 2 tibias (***P < .001).
Absolute requirement of FLT3L but not TSLP in perinatal B-1 lymphopoiesis. (A) FACS profiles and mean (SD) numbers of total lin− AA4.1+B220+CD19+ cells and lin− AA4.1+CD19+B220−/low B-1 progenitors in the BM of 1-day-old WT littermate and Tpte2−/− (n = 8 and n = 6, 2-3 litters) mice. (B) Mean (SD) numbers of total lin−AA4.1+B220+CD19+ cells and B-1 progenitors in 1-day-old BM of littermate Il7−/− (n = 6) and Tpte2−/−Il7−/− (n = 5). (C) FACS profiles and (D) mean (SD) numbers of total lin−AA4.1+B220+CD19+ cells and lin− AA4.1+CD19+B220−/low B-1 progenitors in the BM of 1-day-old WT (n = 16), Flt3l−/− (n = 12), Il7−/− (n = 10), and Flt3l−/−Il7−/− (n = 11) mice (each from at least 2 litters). Numbers in FACS profiles show the percentages of cells within the indicated gates of total BM cells (mean values from all analyzed mice). For panel C, a range of 20 764 to 137 438, 36 500 to 87 584, 22 367 to 74 650, and 16 809 to 143 764 cell events were acquired for the WT, Flt3l−/−, Il7−/−, and Flt3l−/−Il7−/− samples, respectively. # The majority of Flt3l−/−Il7−/− mice had no detectable B-1 progenitors by FACS, and none of the mice had more than 100 cells per 2 tibias (***P < .001).
Discussion
Previous studies have proposed TSLP as the key regulator of fetal and early postnatal IL-7–independent B lymphopoiesis.8,9 Although this conclusion was cast into doubt as more recent studies of fetal B-cell development in mice deficient in IL-7 and TSLPR failed to uncover an essential role of TSLP in IL-7–dependent and –independent fetal B lymphopoiesis,23 the potential importance of TSLP in B lymphopoiesis has been reiterated.25 The establishment of which cytokine(s) might be responsible for IL-7–independent B lymphopoiesis in mice could have important implications for a better understanding of which pathways might regulate human B lymphopoiesis, as this has been suggested to be primarily or even entirely IL-7–independent, although studies performed on patients have been rather restricted.14,16,24
Whereas previous claims in support of a key role of TSLP in IL-7–independent mouse B lymphopoiesis were based on indirect evidence through comparative analysis of B-cell development in Il7- and Il7r-deficient mice and in vitro TSLP responsiveness of B-cell progenitors,6,8,9 the present studies in mice deficient in either one or multiple cytokines provided direct evidence for FLT3L rather than TSLP being essential for IL-7–independent B lymphopoiesis. Thus, although our comparative studies of Il7−/− and Il7r−/− and Il7−/− and Il7−/−Tpte2−/− mice established a limited role of TSLP in IL-7–independent B lymphopoiesis, the importance of TSLP appears to be rather marginal compared with that of FLT3L. Although Tpte2-deficient BM cells and splenocytes have been shown to be unresponsive to stimulation with TSLP ligand,21,22 it cannot be completely ruled out that there might be alternative TSLP receptor signaling subunits at some stages of B-cell development; and, if so, the importance of TSLP in B lymphopoiesis might have been underestimated in our studies. However, importantly, in the absence of FLT3L and IL-7, TSLP was regardless unable to rescue any discernable B-1 or B-2 cell development. Our findings of a lack of a key role of TSLP in mouse B lymphopoiesis are in agreement with the findings in IL-7Rα deficient patients.16,24
Herein, we also identified, for the first time, the role of specific cytokine receptor-ligand pairs in regulation of distinct B-1 B progenitors and found that TSLP was dispensable not only for B-2 B lymphopoiesis but also for IL-7–dependent and -independent regulation of B-1 B progenitors. In contrast, IL-7 and FLT3L play the same critical role in regulation of B-1 B progenitors as of conventional B-2 B progenitors, in that IL-7 appears to be the primary regulator, whereas FLT3L is absolutely required for any IL-7–independent B-1 and B-2 B lymphopoiesis to occur, further demonstrating that TSLP has no ability to rescue early B-cell development in the absence of these 2 regulators of B lymphopoiesis. Thus, these findings demonstrate that, among the investigated cytokines, FLT3L and IL-7 are sufficient and required to drive each of the 2 developmentally distinct pathways of B-1 and B-2 B lymphopoiesis. Importantly, as human severe combined immunodeficiency patients with an IL-2Rg or IL-7Rα deficiency have normal numbers of B cells but abrogated T-cell development, these findings warrant a careful investigation of a potential involvement of FLT3 receptor or ligand dysfunction in human B-cell deficiencies. FLT3L has been shown to facilitate the in vitro generation of human B cells from cord blood progenitors, but it remains to be established to what degree FLT3L is physiologically involved in regulating normal human B lymphopoiesis.35
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lilian Wittman for expert technical assistance, James Ihle (St Jude Children's Research Hospital, Memphis, TN) for generously providing Tpte2−/− mice, Ana Cumano (Pasteur Institute, Paris, France) for kindly providing Il7−/− mice, and Tobias Ryden for help with the statistical analyses.
This study was supported by grants from the Swedish Research Council, Juvenile Diabetes Research Foundation (JDRF; New York, NY), and the Göran Gustafsson Foundation. S.E.W.J. and this project are supported by a Medical Research Council (MRC, London, UK) Strategic Appointment award. The Lund Stem Cell Center is supported by a Center of Excellence grant from the Swedish Foundation for Strategic Research (Stockholm, Sweden).
Authorship
Contribution: C.T.J., E.S., S.K., C.B., and S.E.W.J. designed and conceptualized the research, analyzed the data, and wrote the manuscript; and C.T.J., S.K., C.B., M.C., and A.L. did the phenotypic characterization of different knockout mice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sten Eirik W. Jacobsen, Haematopoietic Stem Cell Laboratory, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, University of Oxford, Headington, Oxford OX3 9DS, United Kingdom; e-mail: sten.jacobsen@imm.ox.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal