Abstract
Mannose-binding lectin (MBL) is a mediator of innate immunity that influences the risk of infection in a range of clinical settings. We previously reported associations between MBL2 genotype and infection in a retrospective study of myeloablative allogeneic hematopoietic stem cell transplantation (allo-HCT). However, other studies have been inconclusive, and the role of MBL in reduced-intensity conditioning (RIC) transplantation is unknown. Here we report a prospective study examining MBL2 genotype, MBL levels, and risk of major infection following HLA-matched sibling myeloablative (n = 83) and RIC (n = 59) HCT. Baseline MBL levels were higher in recipients than donors (P < .001), and recipient MBL levels increased during the peritransplantation period (P = .001), most notably in MBL2 wild-type individuals receiving myeloablative total body irradiation (mTBI). MBL2 coding mutations were associated with major infection in recipients receiving mTBI. The cumulative incidence of major infection in recipient harboring an MBL2 mutation receiving mTBI was 70.6%, compared with 31.1% of those without mutations not receiving mTBI (P = .01). MBL status was not associated with infection in RIC transplants. These results confirm the association of MBL status with risk of infection in myeloablative, TBI-conditioned transplantation. Studies examining the role of MBL replacement therapy to prevent infection in this setting should be considered.
Introduction
The host defense molecule mannose-binding lectin (MBL) is an important mediator of innate immune responses.1 MBL is a multimeric protein predominantly synthesized by the liver that opsonizes a wide range of pathogens independently of antibody and initiates the lectin pathway of complement activation. The basic structural subunit of MBL is a homotrimeric triple helix, and assembly of these subunits into higher order multimers is critical for the normal, complement-fixing activity of MBL. Blood MBL levels vary widely, and much of this variability is explained by several common inherited polymorphisms in the gene encoding MBL, MBL2. Missense mutations in exon 1 at codons 52 (dbSNP rs5030737, Arg→Cys), 54 (rs1800450, Gly→Asp), and 57 (rs1800451, Gly→Glu) disrupt oligomerization of MBL peptides or assembly of MBL multimers. A wild-type coding allele is termed A, and the mutations are termed B (codon 54), C (codon 57), or D (codon 52). The presence of any of these coding mutations is represented by O. Carriage of coding mutations is common, with more than 30% of whites having one coding mutation (A/O), and more than 5% being homozygous or compound heterozygous (O/O).2 The frequency of each coding mutation varies between ethnic groups, with the codon 57 mutation being common in sub–Saharan African populations, but uncommon in whites.3 Additional polymorphisms in the promoter and 5′-untranslated regions of MBL2 at position −550 (rs11003125, G/C, alleles H and L); position −221 (rs7096206, G/C alleles X and Y); and at nucleotide +4 (rs7095891, C/T, alleles P and Q) also influence MBL levels. The −550 and −221 promoter region haplotypes HY, LY, and LX, when in cis with a wild-type coding region (A), are associated with high, intermediate, and low serum MBL levels, respectively.4,5
Mannose-binding lectin status, as assessed either by MBL2 genotyping or measurement of circulating MBL levels, is associated with risk and severity of infection in a broad range of clinical situations, most notably when immune responses are already compromised,1 including following standard6-8 and high-dose9 chemotherapy. In a retrospective study of 96 related, HLA-matched myeloablative transplantations, we detected significant associations between low-producing MBL2 genotypes and increased risk of infection, and the high-producing MBL2 haplotype HYA and reduced risk of major infection.10 An unexpected finding was an independent association with donor MBL2 genotype, suggesting that donor-derived hematopoietic cells may produce clinically significant amounts of MBL following engraftment. As recombinant and purified MBL are under development as therapeutic agents, these findings suggest that prophylactic and/or therapeutic MBL replacement therapy may be indicated to ameliorate the burden of infection in high-risk individuals. However, other retrospective data have been conflicting. Rocha et al found no association between MBL2 exon 1 genotype and infection in the first 100 days after transplantation,11 whereas a recent study identified a significant association between MBL2 genotype and invasive fungal infections after sibling allogeneic hematopoietic stem cell transplantation (allo-HCT).12
These reports suggest that MBL replacement therapy may be useful to reduce the risk of infection after transplantation. However, confirmation of the association between MBL genotype and infection in prospective cohorts, and analysis of the relationship between MBL2 genotype and MBL levels in the allo-HCT setting are required. Furthermore, MBL is primarily synthesized by the liver, and is an acute phase reactant, and the effect of transplantation conditioning regimens, many of which are profoundly hepatotoxic, on peritransplantation MBL levels is unknown. Finally, no studies have examined the role of MBL status and infection in reduced-intensity conditioning (RIC) transplantations. Here we report a prospective study of 142 sibling HLA-matched myeloablative and RIC transplantations incorporating MBL2 genotyping and measurement of peritransplantation MBL levels. MBL status was associated with risk of major infection in recipients undergoing myeloablative total body irradiation (mTBI)–conditioned transplantation.
Methods
Subjects and transplantation characteristics
One hundred forty-two sibling HLA-matched donor-recipient transplant pairs were studied. Transplantations were performed at 4 Australian hospitals (the Royal Adelaide, Royal Melbourne, Alfred, and Westmead hospitals) from 2002 to 2006. All donors and recipients gave informed consent. The study fulfilled the requirements of the Declaration of Helsinki, and was approved by each hospital's Institutional Review Board. Eighty-three recipients received myeloablative conditioning regimens; and 59, reduced-intensity (RIC) regimens. Fifty recipients of myeloablative transplants had received myeloablative TBI (mTBI) at doses of 12 to 13.2 Gy. Patient demographics and transplantation characteristics are shown in Table 1. The stem cell source was peripheral blood in all but 8 cases, and all transplants were T cell replete.
Transplantation characteristics
| . | Myeloablative, no., n = 83 . | Nonmyeloablative, no., n = 59 . |
|---|---|---|
| Mean age at transplantation, y (range, SD) | 39.8 (19-58) | 50.2 (19-64) |
| Recipient sex | 53 M, 30 F | 39 M, 20 F |
| Disease | ||
| ALL | 11 | 0 |
| AML | 43 | 25 |
| CLL | 0 | 3 |
| CML | 7 | 1 |
| HD | 1 | 3 |
| MDS | 1 | 1 |
| MM | 2 | 12 |
| CIMF | 1 | 0 |
| NHL | 11 | 13 |
| PRCA | 1 | 0 |
| SAA | 5 | 0 |
| Renal | 0 | 1 |
| Risk | ||
| Standard | 28 | 18 |
| High | 55 | 41 |
| Stem cell source | ||
| Peripheral blood | 77 | 57 |
| Bone marrow | 6 | 2 |
| Conditioning regimen | ||
| Busulphan plus cyclo | 23 | 0 |
| Busulphan plus cyclo plus etop | 2 | 0 |
| Busulphan plus melphalan | 1 | 0 |
| Cyclo-based chemotherapy* | 6 | 0 |
| Cyclo plus TBI/TNI | 43 | 1 |
| Etoposide plus TBI | 7 | 0 |
| Fludarabine plus cyclo | 1 | 25 |
| Fludarabine plus melphalan | 0 | 24 |
| Melphalan | 0 | 5 |
| Low-dose TBI | 0 | 4 |
| Immunosuppression | ||
| CsA/tacrolimus (± steroid) | 16 | 7 |
| CsA plus MMF (± steroid) | 0 | 6 |
| CsA/tacrolimus plus MTX (± steroid) | 64 | 45 |
| CsA plus MTX plus MMF plus steroid | 3 | 1 |
| Gut decontamination† | ||
| None | 46 | 23 |
| Norfloxacin | 19 | 25 |
| Ciprofloxacin and Metronidazole | 18 | 11 |
| CMV prophylaxis | ||
| Preemptive | 63 | 33 |
| Suppressive | 20 | 26 |
| . | Myeloablative, no., n = 83 . | Nonmyeloablative, no., n = 59 . |
|---|---|---|
| Mean age at transplantation, y (range, SD) | 39.8 (19-58) | 50.2 (19-64) |
| Recipient sex | 53 M, 30 F | 39 M, 20 F |
| Disease | ||
| ALL | 11 | 0 |
| AML | 43 | 25 |
| CLL | 0 | 3 |
| CML | 7 | 1 |
| HD | 1 | 3 |
| MDS | 1 | 1 |
| MM | 2 | 12 |
| CIMF | 1 | 0 |
| NHL | 11 | 13 |
| PRCA | 1 | 0 |
| SAA | 5 | 0 |
| Renal | 0 | 1 |
| Risk | ||
| Standard | 28 | 18 |
| High | 55 | 41 |
| Stem cell source | ||
| Peripheral blood | 77 | 57 |
| Bone marrow | 6 | 2 |
| Conditioning regimen | ||
| Busulphan plus cyclo | 23 | 0 |
| Busulphan plus cyclo plus etop | 2 | 0 |
| Busulphan plus melphalan | 1 | 0 |
| Cyclo-based chemotherapy* | 6 | 0 |
| Cyclo plus TBI/TNI | 43 | 1 |
| Etoposide plus TBI | 7 | 0 |
| Fludarabine plus cyclo | 1 | 25 |
| Fludarabine plus melphalan | 0 | 24 |
| Melphalan | 0 | 5 |
| Low-dose TBI | 0 | 4 |
| Immunosuppression | ||
| CsA/tacrolimus (± steroid) | 16 | 7 |
| CsA plus MMF (± steroid) | 0 | 6 |
| CsA/tacrolimus plus MTX (± steroid) | 64 | 45 |
| CsA plus MTX plus MMF plus steroid | 3 | 1 |
| Gut decontamination† | ||
| None | 46 | 23 |
| Norfloxacin | 19 | 25 |
| Ciprofloxacin and Metronidazole | 18 | 11 |
| CMV prophylaxis | ||
| Preemptive | 63 | 33 |
| Suppressive | 20 | 26 |
Standard risk was defined as acute myeloid leukemia in first remission and chronic myeloid leukemia in first chronic phase. All other diseases and stages were considered high risk.
Cyclo indicates cyclophosphamide; Etop, etoposide; TBI, total body irradiation; TNI, total nodal irradiation; CsA, cyclosporine A; MMF, mycophenolate mofetil; and MTX, methotrexate.
Includes cyclophosphamide alone (n = 3), or with ATG (n = 2) or lomustine, cytarabine and etoposide (n = 1).
Ciprofloxacin 750 mg twice daily, metronidazole 400 mg daily, norfloxacin 400 mg twice daily. Suppressive CMV prophylaxis was with regular intravenous ganciclovir or oral valaciclovir.
Comprehensive clinical data were collected for each recipient for the first 100 days after transplantation. Major infection was defined as systemic or invasive episodes of sepsis requiring clinical intervention confirmed on microbiologic testing, or with highly suggestive radiographic features in which microbiologic testing was not possible or inconclusive.10 Single positive blood culture results for skin commensals, venous catheter exit site infections, local reactivation of herpes simplex, and asymptomatic positivity for Clostridium difficile were excluded. In contrast to our previous study, in which most patients received cytomegalovirus (CMV) prophylaxis with intravenous ganciclovir,10 many recipients in the current study received no CMV prophylaxis, but were treated preemptively with ganciclovir or foscarnet upon rising blood CMV DNA levels. CMV reactivation was thus considered significant only if symptomatic or associated with organ dysfunction. Episodes of asymptomatic CMV reactivation were excluded. All results were reviewed by the local bone marrow transplant physician and one of the authors (P.G.B or U.H.). Additional clinical data collected included overall and event-free survival, graft-versus-host disease (GVHD, graded according to revised Glucksberg criteria), duration of neutropenia, and fever.
MBL2 genotyping and MBL level measurement
Five polymorphisms in the MBL2 gene were genotyped including those at −550, −221, and codons 52, 54, and 57 using the polymerase chain reaction and sequence-specific primers as previously described.13 Genotyping was successfully performed for 136 donor-recipient pairs. MBL levels were quantitated by 2 assays that measure the functional activity of multimeric, complement-fixing MBL, as previously described2 : binding of MBL to solid-phase mannan (mannan-binding assay), and complement-fixing activity as measured by C4 deposition (C4-deposition assay). Measurement of antigenic MBL levels by enzyme-linked immunosorbent assay (ELISA) may detect non–complement-fixing, lower molecular weight oligomers of MBL, and was not performed.2 MBL levels were measured prior to commencement of pretransplantation conditioning (for recipients) and at predonation assessment (for donors), and at days 0, 14, and 28 after transplantation for recipients. MBL assays were performed on plasma subjected to no more than one freeze-thaw cycle.
Statistical analyses
Associations between categoric variables were analyzed using Fisher exact test. Analysis of temporal changes in MBL levels during the peritransplantation period was performed using repeated measures analysis of variance. Unpaired associations between categoric and continuous variables were analyzed using the Student t test where assumptions for parametric testing were met, otherwise the Mann-Whitney test was used. All P values reported are 2 tailed. Estimation of the cumulative incidences of major infection, acute graft-versus-host disease (GVHD), and grades II to IV GVHD with consideration of competing events was performed using Gray's estimator.14 Noninfective death and non–GVHD-related deaths were treated as competing risks for analyses of the incidence of major infection and GVHD, respectively. Multivariable analyses of associations with infection and GVHD with consideration of competing risks were performed using the method of Fine and Gray.15 For each multivariable analysis, all variables with univariate P values less than .25 were included in the initial model, and then variables with P values more than .1 were sequentially removed, in order of P value (highest to lowest). Multicolinear variables (eg, MBL2 coding mutations and MBL2-“insufficient” genotypes) were handled by including only the variable with most significant univariate P value in the multivariable model.
The following clinical variables were examined in univariate analyses of infection risk: age at transplantation; disease (AML vs other); disease status (standard or high risk); the combination of female donor and male recipient; myeloablative versus RIC conditioning regimens; myeloablative total body irradiation; immunosuppressive regimen; stem cell source; gut decontamination regimen; donor and recipient cytomegalovirus serostatus; duration of neutropenia; and acute GVHD (any grade, grades II-IV, and grades III,IV). The following MBL variables for recipient and donor were examined in univariate analyses: the carriage of MBL2 coding mutations, MBL2 coding genotype (A/A, A/O, O/O), MBL2 −550 (H/L) and −221 (X/Y) promoter genotypes, and MBL2-insufficient genotypes (defined below); baseline mannan-binding and C4-deposition levels; maximal levels during the baseline to day-28 measurement period; and the maximal rise from baseline to peak MBL level during the measurement period. Two definitions of MBL2-insufficient genotypes were used in these analyses: (1) LXA homozygosity or the presence of MBL2 coding mutations; and (2) LXA homozygosity or the presence of MBL2 coding mutations with the exception of HYD/A.
Analyses were performed using JMP 7.0 (SAS, Cary, NC), Prism 5.0 (GraphPad Software, San Diego, CA), and the cmprsk and CumIncidence16 packages in R (The R Foundation, http://www.R-project.org).
Results
MBL2 genotype and MBL levels
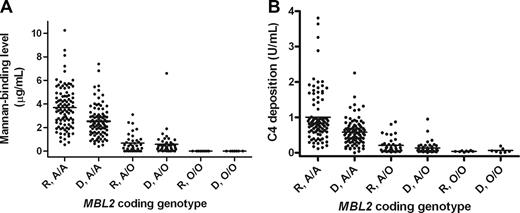
Fifty-one (37.5%) donors and 44 (32.3%) recipients carried an MBL2 coding mutation (donor vs recipient, P = .446). These frequencies are comparable to previously reported white populations.2 As expected, the presence of MBL2 coding mutations was associated with significantly reduced baseline MBL levels, in both donor and recipients, as measured by both mannan-binding and C4-deposition assays (Table 2; Figure 1). In recipients, the mean (± SE) baseline mannan-binding MBL level in MBL2 coding heterozygotes (A/O) was 0.68 (± 0.13) mg/mL, compared with 3.69 (± 0.18) for wild-type (A/A) recipients (P < .001). Negligible levels were observed in coding mutation homozygotes (O/O). Similar differences were observed in donors (A/A, 2.53 ± 0.15 vs O/O, 0.58 ± 0.15; P < .001), and for MBL C4-deposition levels (Table 2). A comprehensive listing of full MBL2 haplotypic combinations and associated MBL levels is provided in Table 2 and in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). As previously reported,4 the MBL2 52D coding mutation was associated with less markedly reduced MBL levels than the other MBL2 coding mutations.
MBL2 coding genotype frequencies and baseline MBL levels in donors and recipients
| MBL2 genotype . | Mannan binding . | C4-deposition levels . | ||||||
|---|---|---|---|---|---|---|---|---|
| Donor . | Recipient . | P . | Donor . | Recipient . | P . | |||
| n . | Mean plus or minus SD, μg/mL (range) . | n . | Mean plus or minus SD, μg/mL (range) . | Mean plus or minus SD, U/mL (range) . | Mean plus or minus SD, U/mL (range) . | |||
| A/A | 85 | 2.53 ± 1.36 (0.40-7.4) | 92 | 3.69 ± 1.81 (0.54-10.25) | <.001 | 0.58 ± 0.36 (0-2.25) | 1.01 ± 0.70 (0.12-3.81) | <.001 |
| A/O | 44 | 0.58 ± 1.023 (0-6.59) | 36 | 0.68 ± 0.78 (0-3.10) | NS | 0.14 ± 0.17 (0.01-0.95) | 0.21 ± 0.22 (0.02-0.87) | .095 |
| A/B | 30 | 0.52 ± 1.18 (0-6.59) | 21 | 0.39 ± 0.32 (0-1.01) | NS | 0.11 ± 0.17 (0.01-0.95) | 0.12 ± 0.07 (0.02-0.24) | NS |
| A/C | 1 | 1.47 | 3 | 0.03 ± 0.04 (0-0.08) | 0.23 | 0.06 ± 0.03 (0.03-0.08) | ||
| A/D | 13 | 0.65 ± 0.55 (0-1.89) | 12 | 1.35 ± 1.00 (0-3.10) | .038 | 0.18 ± 0.17 (0.03-0.61) | 0.41 ± 0.28 (0.03-0.87) | .019 |
| O/O | 7 | 0 ± 0 (0-0) | 8 | 0 ± 0 (0-0) | NS | 0.07 ± 0.07 (0.0-0.19) | 0.04 ± 0.02 (0.01-0.07) | NS |
| YA/YA | 50 | 3.11 ± 1.35 (0.89-7.39) | 54 | 4.10 ± 1.64 (1.46-8.57) | .001 | 0.70 ± 0.40 (0-2.25) | 1.00 ± 0.64 (0.20-3.64) | .005 |
| YA/XA | 32 | 1.88 ± 0.91 (0.51-4.84) | 32 | 3.49 ± 1.81 (1.16-10.25) | <.001 | 0.44 ± 0.22 (0.03-0.92) | 1.15 ± 0.79 (0.30-3.81) | <.001 |
| YA/O | 33 | 0.74 ± 1.14 (0-6.59) | 26 | 0.87 ± 0.82 (0.0-3.1) | NS | 0.16 ± 0.18 (0.03-0.95) | 0.35 ± 0.23 (0.04-0.87) | .092 |
| XA/XA | 3 | 0.61 ± 0.21 (0.40-0.81) | 6 | 1.20 ± 0.77 (0.54-2.64) | NS | 0.15 ± 0.04 (0.13-0.2) | 0.30 ± 0.25 (0.12-0.78) | NS |
| XA/O | 11 | 0.10 ± 0.16 (0-0.51 | 10 | 0.18 ± 0.40 (0-1.29) | NS | 0.06 ± 0.03 (0.01-0.10) | 0.09 ± 0.17 (0.02-0.56) | NS |
| HYA/HYA | 15 | 3.11 ± 1.37 (1.33-6.8) | 20 | 3.96 ± 1.46 (1.97-6.78) | .082 | 0.75 ± 0.53 (0-2.25) | 0.87 ± 0.43 (0.27-1.76) | NS |
| HYA/LYA | 30 | 3.18 ± 1.40 (0.89-7.40) | 30 | 4.24 ± 1.79 (1.46-8.57) | .013 | 0.70 ± 0.33 (0.11-1.58) | 1.11 ± 0.79 (0.20-3.64) | .011 |
| HYA/LXA | 19 | 1.92 ± 0.92 (0.82-4.84) | 16 | 3.33 ± 1.30 (1.16-5.37) | .001 | 0.46 ± 0.22 (0.11-0.92) | 1.20 ± 0.86 (0.36-3.81) | .001 |
| HYA/B | 12 | 0.52 ± 0.32 (0.03-1.26) | 12 | 0.52 ± 0.34 (0-1.01) | NS | 0.12 ± 0.06 (0.03-0.22) | 0.14 ± 0.07 (0.04-0.23) | NS |
| HYA/C | 1 | 1.45 | 0 | 0.233 | ||||
| HYA/D | 9 | 0.84 ± 0.54 (0-1.89) | 4 | 1.83 ± 0.93 (0.91-3.10) | .032 | 0.23 ± 0.18 (0.03-0.61) | 0.50 ± 0.27 (0.26-0.87) | .057 |
| LYA/LYA | 6 | 2.50 ± 1.02 (1.01-3.92) | 5 | 3.51 ± 1.43 (1.91-5.68) | NS | 0.56 ± 0.33 (0.29-1.20) | 0.84 ± 0.09 (0.73-0.97) | .100 |
| LYA/LXA | 14 | 1.77 ± 0.91 (0.51-3.83) | 17 | 3.66 ± 2.21 (1.48-10.25) | .006 | 0.41 ± 0.23 (0.04-0.78) | 1.1 ± 0.74 (0.30-3.36) | .003 |
| LYA/B | 11 | 0.83 ± 1.92 (0.06-6.59 | 5 | 0.34 ± 0.15 (0.19-0.55) | NS | 0.15 ± 0.27 (0.03-0.95) | 0.12 ± 0.08 (0.05-0.24) | NS |
| LYA/D | 0 | 5 | 1.48 ± 1.00 (0-2.43) | 0.46 ± 0.28 (0.05-0.80 | ||||
| LXA/LXA | 3 | 0.61 ± 0.21 (0.40-0.81) | 6 | 1.2 ± 0.77 (0.54-2.64) | NS | 0.15 ± 0.04 (0.13-0.20) | 0.30 ± 0.25 (0.12-0.78) | NS |
| LXA/B | 7 | 0.04 ± 0.06 (0.0-0.16) | 5 | 0.05 ± 0.11 (0-0.24) | NS | 0.05 ± 0.03 (0.01-0.08) | 0.04 ± 0.01 (0.02-0.06) | NS |
| LXA/C | 0 | 2 | 0.04 ± 0.06 (0-0.08) | 0.05 ± 0.04 (0.03-0.08) | ||||
| LXA/D | 4 | 0.22 ± 0.22 (0.0-0.51) | 3 | 0.50 ± 0.69 (0.01-1.29) | NS | 0.07 ± 0.03 (0.04-0.10) | 0.211 ± 0.31 (0.03-0.56) | NS |
| MBL2 genotype . | Mannan binding . | C4-deposition levels . | ||||||
|---|---|---|---|---|---|---|---|---|
| Donor . | Recipient . | P . | Donor . | Recipient . | P . | |||
| n . | Mean plus or minus SD, μg/mL (range) . | n . | Mean plus or minus SD, μg/mL (range) . | Mean plus or minus SD, U/mL (range) . | Mean plus or minus SD, U/mL (range) . | |||
| A/A | 85 | 2.53 ± 1.36 (0.40-7.4) | 92 | 3.69 ± 1.81 (0.54-10.25) | <.001 | 0.58 ± 0.36 (0-2.25) | 1.01 ± 0.70 (0.12-3.81) | <.001 |
| A/O | 44 | 0.58 ± 1.023 (0-6.59) | 36 | 0.68 ± 0.78 (0-3.10) | NS | 0.14 ± 0.17 (0.01-0.95) | 0.21 ± 0.22 (0.02-0.87) | .095 |
| A/B | 30 | 0.52 ± 1.18 (0-6.59) | 21 | 0.39 ± 0.32 (0-1.01) | NS | 0.11 ± 0.17 (0.01-0.95) | 0.12 ± 0.07 (0.02-0.24) | NS |
| A/C | 1 | 1.47 | 3 | 0.03 ± 0.04 (0-0.08) | 0.23 | 0.06 ± 0.03 (0.03-0.08) | ||
| A/D | 13 | 0.65 ± 0.55 (0-1.89) | 12 | 1.35 ± 1.00 (0-3.10) | .038 | 0.18 ± 0.17 (0.03-0.61) | 0.41 ± 0.28 (0.03-0.87) | .019 |
| O/O | 7 | 0 ± 0 (0-0) | 8 | 0 ± 0 (0-0) | NS | 0.07 ± 0.07 (0.0-0.19) | 0.04 ± 0.02 (0.01-0.07) | NS |
| YA/YA | 50 | 3.11 ± 1.35 (0.89-7.39) | 54 | 4.10 ± 1.64 (1.46-8.57) | .001 | 0.70 ± 0.40 (0-2.25) | 1.00 ± 0.64 (0.20-3.64) | .005 |
| YA/XA | 32 | 1.88 ± 0.91 (0.51-4.84) | 32 | 3.49 ± 1.81 (1.16-10.25) | <.001 | 0.44 ± 0.22 (0.03-0.92) | 1.15 ± 0.79 (0.30-3.81) | <.001 |
| YA/O | 33 | 0.74 ± 1.14 (0-6.59) | 26 | 0.87 ± 0.82 (0.0-3.1) | NS | 0.16 ± 0.18 (0.03-0.95) | 0.35 ± 0.23 (0.04-0.87) | .092 |
| XA/XA | 3 | 0.61 ± 0.21 (0.40-0.81) | 6 | 1.20 ± 0.77 (0.54-2.64) | NS | 0.15 ± 0.04 (0.13-0.2) | 0.30 ± 0.25 (0.12-0.78) | NS |
| XA/O | 11 | 0.10 ± 0.16 (0-0.51 | 10 | 0.18 ± 0.40 (0-1.29) | NS | 0.06 ± 0.03 (0.01-0.10) | 0.09 ± 0.17 (0.02-0.56) | NS |
| HYA/HYA | 15 | 3.11 ± 1.37 (1.33-6.8) | 20 | 3.96 ± 1.46 (1.97-6.78) | .082 | 0.75 ± 0.53 (0-2.25) | 0.87 ± 0.43 (0.27-1.76) | NS |
| HYA/LYA | 30 | 3.18 ± 1.40 (0.89-7.40) | 30 | 4.24 ± 1.79 (1.46-8.57) | .013 | 0.70 ± 0.33 (0.11-1.58) | 1.11 ± 0.79 (0.20-3.64) | .011 |
| HYA/LXA | 19 | 1.92 ± 0.92 (0.82-4.84) | 16 | 3.33 ± 1.30 (1.16-5.37) | .001 | 0.46 ± 0.22 (0.11-0.92) | 1.20 ± 0.86 (0.36-3.81) | .001 |
| HYA/B | 12 | 0.52 ± 0.32 (0.03-1.26) | 12 | 0.52 ± 0.34 (0-1.01) | NS | 0.12 ± 0.06 (0.03-0.22) | 0.14 ± 0.07 (0.04-0.23) | NS |
| HYA/C | 1 | 1.45 | 0 | 0.233 | ||||
| HYA/D | 9 | 0.84 ± 0.54 (0-1.89) | 4 | 1.83 ± 0.93 (0.91-3.10) | .032 | 0.23 ± 0.18 (0.03-0.61) | 0.50 ± 0.27 (0.26-0.87) | .057 |
| LYA/LYA | 6 | 2.50 ± 1.02 (1.01-3.92) | 5 | 3.51 ± 1.43 (1.91-5.68) | NS | 0.56 ± 0.33 (0.29-1.20) | 0.84 ± 0.09 (0.73-0.97) | .100 |
| LYA/LXA | 14 | 1.77 ± 0.91 (0.51-3.83) | 17 | 3.66 ± 2.21 (1.48-10.25) | .006 | 0.41 ± 0.23 (0.04-0.78) | 1.1 ± 0.74 (0.30-3.36) | .003 |
| LYA/B | 11 | 0.83 ± 1.92 (0.06-6.59 | 5 | 0.34 ± 0.15 (0.19-0.55) | NS | 0.15 ± 0.27 (0.03-0.95) | 0.12 ± 0.08 (0.05-0.24) | NS |
| LYA/D | 0 | 5 | 1.48 ± 1.00 (0-2.43) | 0.46 ± 0.28 (0.05-0.80 | ||||
| LXA/LXA | 3 | 0.61 ± 0.21 (0.40-0.81) | 6 | 1.2 ± 0.77 (0.54-2.64) | NS | 0.15 ± 0.04 (0.13-0.20) | 0.30 ± 0.25 (0.12-0.78) | NS |
| LXA/B | 7 | 0.04 ± 0.06 (0.0-0.16) | 5 | 0.05 ± 0.11 (0-0.24) | NS | 0.05 ± 0.03 (0.01-0.08) | 0.04 ± 0.01 (0.02-0.06) | NS |
| LXA/C | 0 | 2 | 0.04 ± 0.06 (0-0.08) | 0.05 ± 0.04 (0.03-0.08) | ||||
| LXA/D | 4 | 0.22 ± 0.22 (0.0-0.51) | 3 | 0.50 ± 0.69 (0.01-1.29) | NS | 0.07 ± 0.03 (0.04-0.10) | 0.211 ± 0.31 (0.03-0.56) | NS |
MBL2 genotypic data have been shown as coding genotypes (A indicates coding wild-type; O, coding mutation; and B, C, and D, each of the coding mutations), haplotypes of the MBL2 −221 promoter polymorphism (X/Y) and coding genotype (A or O), and full promoter-coding MBL2 haplotypes, where −550 H/L represents the second promoter polymorphism, and HYA, LYA, and LXA result in high, intermediate, and low levels of MBL, respectively. MBL levels were measured by 2 assays: binding of MBL to solid phase mannan (Mannan binding) and complement fixation (C4-deposition). P values report comparisons of MBL levels for each genotype or haplotype between donors and recipients.
NS indicates not significant.
Baseline MBL levels in donors and recipients stratified by MBL2 coding genotype. A wild-type MBL2 coding region is A and a region with a mutation is O, thus A/A denotes a wild-type homozygote; A/O, a heterozygote; and O/O, a mutation homozygote. (A) Mannan-binding recipient (R) versus donor (D) A/A; Mann-Whitney, P < .001. R versus D A/O; P = NS. (B) C4-deposition recipient (R) versus donor (D) A/A; P < .001. R versus D A/O; P = not significant.
Baseline MBL levels in donors and recipients stratified by MBL2 coding genotype. A wild-type MBL2 coding region is A and a region with a mutation is O, thus A/A denotes a wild-type homozygote; A/O, a heterozygote; and O/O, a mutation homozygote. (A) Mannan-binding recipient (R) versus donor (D) A/A; Mann-Whitney, P < .001. R versus D A/O; P = NS. (B) C4-deposition recipient (R) versus donor (D) A/A; P < .001. R versus D A/O; P = not significant.
Baseline MBL levels were significantly higher in recipients than donors (Figure 1; Figure S1). This was significant for MBL2 coding wild-type (A/A) cases in both mannan-binding and C4-deposition assays (P < .001), and also for A/D individuals (P = .019). Significant differences were also seen for YA/YA and YA/XA individuals (Table 2). These differences likely reflect an acute phase response induced by disease, illness, and prior therapy.
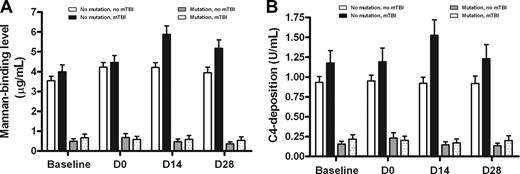
MBL levels rose significantly during the peritransplantation period. Examining the entire cohort that underwent transplantation, mean mannan-binding MBL levels rose from 2.69 μg/mL at baseline to a maximum of 3.40 μg/mL at day 14, and fell to 3.07 at day 28. A nonsignificant increase was observed for C4-deposition levels (baseline mean, 0.75 U/mL to 0.82 at day 14). These increases were primarily observed in recipients lacking MBL2 coding mutations (A/A genotype; mean rise from baseline to day 14, 0.68 μg/mL; P < .001), or who received myeloablative TBI as part of pretransplantation conditioning (mean baseline to day 14 rise, 1.19 mg/mL; P = .010). The rise in MBL levels was maximal in patients who received TBI and also lacked MBL2 mutations (mean rise, 1.90 μg/mL; P < .05). Similarly, MBL levels as measured by C4 deposition also rose in TBI-conditioned, MBL2 A/A individuals from baseline to day 14 (mean, 1.18 to 1.52 U/mL; Figure 2B).
Recipient MBL levels (mannan binding) during the peritransplantation period. Recipient MBL mannan-binding (A) and C4-deposition (B) levels are shown stratified by recipient MBL2 coding genotype and the use of myeloablative total body irradiation in the conditioning regimen. There is a significant increase in MBL levels most evident in recipients lacking MBL2 coding mutations who received mTBI. The increase is maximal from baseline (prior to commencement of conditioning) to the 14th day after transplantation. Bars show mean plus SEM.
Recipient MBL levels (mannan binding) during the peritransplantation period. Recipient MBL mannan-binding (A) and C4-deposition (B) levels are shown stratified by recipient MBL2 coding genotype and the use of myeloablative total body irradiation in the conditioning regimen. There is a significant increase in MBL levels most evident in recipients lacking MBL2 coding mutations who received mTBI. The increase is maximal from baseline (prior to commencement of conditioning) to the 14th day after transplantation. Bars show mean plus SEM.
Associations with infection
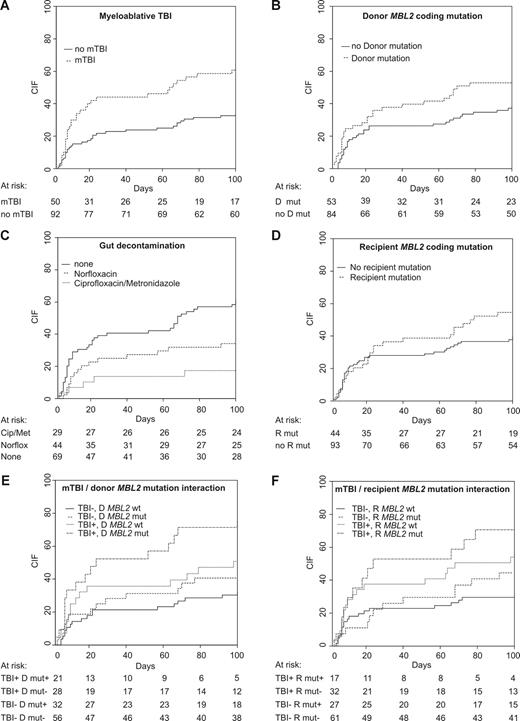
One-hundred–day transplant-related mortality was 9.2% (myeloablative, 9.3%; RIC, 8.5%). Four recipients died of infection-related causes (3 myeloablative and 1 RIC) and 5, of GVHD-related causes (3 myeloablative and 2 RIC). Sixty (42.3%) transplant recipients experienced a total of 93 episodes of clinically significant infection in the first 100 days after transplantation. Thirty-six recipients had infections prior to neutrophil count recovery (41 episodes), and 35 recipients had a total of 52 episodes following recovery. Fifty-five recipients had at least one episode of bacterial sepsis, the majority of which were episodes of bacteremia (52 recipients). Six recipients experienced fungal infections and 10, viral infections. In univariate analysis, myeloablative TBI (mTBI; CIF, 62.7% vs 33.7%; P = .001; Table 3; Figure 3A), the absence of gut decontamination (61.4% vs 34.8%; P = .003; Figure 3C), and steroid-containing immunosuppression (57.1% vs 38.6%; P = .039) were associated with an increased risk of major infection in the first 100 days after transplantation.
Factors associated with major infection and GVHD
| . | Major infection . | Acute GVHD grades I-IV . | Acute GVHD grades II-IV . | |||
|---|---|---|---|---|---|---|
| CIF plus or minus SE . | P . | CIF plus or minus SE . | P . | CIF plus or minus SE . | P . | |
| Disease risk | .035 | .981 | .382 | |||
| Standard | 30.4 ± 6.9 | 50 ± 7.5 | 26.1 ± 6.6 | |||
| High | 50.3 ± 5.2 | 50.3 ± 5.1 | 33.6 ± 4.9 | |||
| Female donor, male recipient | .87 | .456 | .065 | |||
| No | 44.4 ± 4.8 | 52.2 ± 4.8 | 35. 1 ± 4.6 | |||
| Yes | 41.9 ± 9.1 | 42.9 ± 9.3 | 16.4 ± 6.8 | |||
| Stem cell source | .49 | .012 | .005 | |||
| Bone marrow | 62.5 ± 19.2 | 87.5 ± 15.4 | 75.0 ± 17.9 | |||
| Peripheral blood | 42.7 ± 4.3 | 48.0 ± 4.4 | 28. 5 ± 3.9 | |||
| Conditioning | .232 | .230 | .920 | |||
| Reduced intensity | 37.3 ± 6.4 | 46.4 ± 6.6 | 32.7 ± 6.2 | |||
| Myeloablative | 48.5 ± 5.6 | 53 ± 5.5 | 30.1 ± 5 | |||
| mTBI | .001 | .999 | .727 | |||
| No mTBI | 33.7 ± 5.0 | 51.5 ± 5.3 | 30.7 ± 4.9 | |||
| mTBI | 62.7 ± 7.0 | 48 ± 7.2 | 32 ± 6.7 | |||
| Decontamination | .001 | .040 | .501 | |||
| None | 61.4 ± 6.0 | 42 ± 6 | 27.5 ± 5.4 | |||
| Norfloxacin | 34.1 ± 7.3 | 52.3 ± 7.7 | 32.1 ± 7.2 | |||
| Ciprofloxacin & metronidazole | 17.2 ± 7.1 | 65.5 ± 9.1 | 37.9 ± 9.2 | |||
| Decontamination | .003 | .007 | .050 | |||
| None | 61.4 ± 6.0 | 38.0 ± 7.0 | 22 ± 5.9 | |||
| Any | 34.8 ± 5.0 | 57.0 ± 5.2 | 36.1 ± 5.1 | |||
| Immunosuppression | .039 | .241 | .21 | |||
| No steroid | 38.6 ± 4.9 | 52.9 ± 5 | 33.9 ± 4.8 | |||
| Steroid containing | 57.1 ± 8.2 | 43.6 ± 0.08 | 23.1 ± 6.8 | |||
| Recipient mutation | .17 | .102 | .51 | |||
| No mutation | 40.0 ± 5.1 | 47.7 ± 5.2 | 30.3 ± 4.8 | |||
| Mutation | 54.5 ± 7.6 | 59 ± 7.6 | 34.1 ± 7.2 | |||
| Recipient MBL2 insufficiency | .665 | .046 | .043 | |||
| Sufficient | 45.8 ± 4.8 | 47.6 ± 5.8 | 27.8 ± 4.3 | |||
| Insufficient | 40.0 ± 10.1 | 68 ± 9.7 | 48 ± 10.3 | |||
| Donor mutation | .041 | .460 | .872 | |||
| No mutation | 38.3 ± 5.4 | 48.1 ± 5.5 | 32.4 ± 5.2 | |||
| Mutation | 54.7 ± 6.9 | 54.7 ± 6.9 | 30.2 ± 6.4 | |||
| Donor MBL2-insufficient genotypes | .63 | .525 | .751 | |||
| Sufficient | 43.1 ± 5.2 | 48.8 ± 5.3 | 31 ± 4.9 | |||
| Insufficient | 47.8 ± 7.5 | 54.3 ± 7.5 | 32.6 ± 7 | |||
| . | Major infection . | Acute GVHD grades I-IV . | Acute GVHD grades II-IV . | |||
|---|---|---|---|---|---|---|
| CIF plus or minus SE . | P . | CIF plus or minus SE . | P . | CIF plus or minus SE . | P . | |
| Disease risk | .035 | .981 | .382 | |||
| Standard | 30.4 ± 6.9 | 50 ± 7.5 | 26.1 ± 6.6 | |||
| High | 50.3 ± 5.2 | 50.3 ± 5.1 | 33.6 ± 4.9 | |||
| Female donor, male recipient | .87 | .456 | .065 | |||
| No | 44.4 ± 4.8 | 52.2 ± 4.8 | 35. 1 ± 4.6 | |||
| Yes | 41.9 ± 9.1 | 42.9 ± 9.3 | 16.4 ± 6.8 | |||
| Stem cell source | .49 | .012 | .005 | |||
| Bone marrow | 62.5 ± 19.2 | 87.5 ± 15.4 | 75.0 ± 17.9 | |||
| Peripheral blood | 42.7 ± 4.3 | 48.0 ± 4.4 | 28. 5 ± 3.9 | |||
| Conditioning | .232 | .230 | .920 | |||
| Reduced intensity | 37.3 ± 6.4 | 46.4 ± 6.6 | 32.7 ± 6.2 | |||
| Myeloablative | 48.5 ± 5.6 | 53 ± 5.5 | 30.1 ± 5 | |||
| mTBI | .001 | .999 | .727 | |||
| No mTBI | 33.7 ± 5.0 | 51.5 ± 5.3 | 30.7 ± 4.9 | |||
| mTBI | 62.7 ± 7.0 | 48 ± 7.2 | 32 ± 6.7 | |||
| Decontamination | .001 | .040 | .501 | |||
| None | 61.4 ± 6.0 | 42 ± 6 | 27.5 ± 5.4 | |||
| Norfloxacin | 34.1 ± 7.3 | 52.3 ± 7.7 | 32.1 ± 7.2 | |||
| Ciprofloxacin & metronidazole | 17.2 ± 7.1 | 65.5 ± 9.1 | 37.9 ± 9.2 | |||
| Decontamination | .003 | .007 | .050 | |||
| None | 61.4 ± 6.0 | 38.0 ± 7.0 | 22 ± 5.9 | |||
| Any | 34.8 ± 5.0 | 57.0 ± 5.2 | 36.1 ± 5.1 | |||
| Immunosuppression | .039 | .241 | .21 | |||
| No steroid | 38.6 ± 4.9 | 52.9 ± 5 | 33.9 ± 4.8 | |||
| Steroid containing | 57.1 ± 8.2 | 43.6 ± 0.08 | 23.1 ± 6.8 | |||
| Recipient mutation | .17 | .102 | .51 | |||
| No mutation | 40.0 ± 5.1 | 47.7 ± 5.2 | 30.3 ± 4.8 | |||
| Mutation | 54.5 ± 7.6 | 59 ± 7.6 | 34.1 ± 7.2 | |||
| Recipient MBL2 insufficiency | .665 | .046 | .043 | |||
| Sufficient | 45.8 ± 4.8 | 47.6 ± 5.8 | 27.8 ± 4.3 | |||
| Insufficient | 40.0 ± 10.1 | 68 ± 9.7 | 48 ± 10.3 | |||
| Donor mutation | .041 | .460 | .872 | |||
| No mutation | 38.3 ± 5.4 | 48.1 ± 5.5 | 32.4 ± 5.2 | |||
| Mutation | 54.7 ± 6.9 | 54.7 ± 6.9 | 30.2 ± 6.4 | |||
| Donor MBL2-insufficient genotypes | .63 | .525 | .751 | |||
| Sufficient | 43.1 ± 5.2 | 48.8 ± 5.3 | 31 ± 4.9 | |||
| Insufficient | 47.8 ± 7.5 | 54.3 ± 7.5 | 32.6 ± 7 | |||
Univariate associations with infection, incorporating consideration of noninfective death as a competing risk, are shown.
CIF indicates cumulative incidence fraction; SE, standard error; and mTBI, myeloablative TBI.
Cumulative incidence of infection stratified according to transplantation characteristics or MBL2 status. Cip indicates ciprofloxacin; D, donor; Met, metronidazole; mut, MBL2 coding mutation; mTBI, myeloablative TBI; Norflox, norfloxacin; R, recipient; and wt, MBL2 coding wild type.
Cumulative incidence of infection stratified according to transplantation characteristics or MBL2 status. Cip indicates ciprofloxacin; D, donor; Met, metronidazole; mut, MBL2 coding mutation; mTBI, myeloablative TBI; Norflox, norfloxacin; R, recipient; and wt, MBL2 coding wild type.
Analyzing the entire cohort that underwent transplantation, we found that both donor and recipient MBL2 exon 1 mutations (A/O or O/O genotypes) were weak risk factors for infection (recipient genotype, P = .17; donor genotype, P = .041; Table 3; Figure 3B,D). In contrast, incorporation of other reportedly “low-producing” MBL2 genotypes into a combined MBL-insufficient risk factor did not increase the strength of association with infection risk—only MBL2 coding mutations were associated with an increased cumulative incidence of infection. Importantly, the association between MBL2 genotype and infection risk was dependent on transplantation type, with significant interaction observed between MBL2 coding genotype and the use of mTBI. The cumulative incidence of major infection in recipients with MBL2 mutations who received mTBI was 70.6%, compared with 31.1% lacking a MBL2 coding mutation and not receiving mTBI (Table 4; Figure 3F). A similar interaction was observed for donor MBL2 coding genotype and mTBI: 76.2% of recipients undergoing mTBI and receiving grafts from MBL2 coding mutation–positive donors experienced infection, compared with 31.1% of recipients who did not receive TBI with MBL2 A/A donors (P = .013; Table 4; Figure 3E).
Interaction of MBL2 coding genotype and myeloablative TBI and outcome following transplantation
| . | Major infection . | Acute GVHD . | Grades II-IV acute GVHD . | |||
|---|---|---|---|---|---|---|
| CIF plus or minus SE . | P . | CIF plus or minus SE . | P . | CIF plus or minus SE . | P . | |
| Interaction of mTBI and donor MBL2 mutation | .003 | .750 | .916 | |||
| mTBI−, D MBL2 wt | 32.1 ± 6.3 | 47.1 ± 6.8 | 30.8 ± 6.3 | |||
| mTBI−, D mutation | 40.6 ± 8.9 | 59.4 ± 8.9 | 31.3 ± 8.4 | |||
| mTBI+, D MBL2 wt | 50.8 ± 9.8 | 50.0 ± 9.7 | 35.7 ± 9.3 | |||
| mTBI+, D mutation | 76.2 ± 10.0 | 47.6 ± 11.3 | 28.6 ± 10.2 | |||
| Interaction of mTBI and recipient MBL2 mutation | .013 | .407 | .636 | |||
| mTBI−, R MBL2 wt | 31.1 ± 6.0 | 49.9 ± 6.5 | 31.6 ± 6.1 | |||
| mTBI−, R mutation | 44.4 ± 9.8 | 59.3 ± 9.8 | 29.6 ± 9.0 | |||
| mTBI+, R MBL2 wt | 57.2 ± 9.2 | 43.8 ± 9.0 | 28.1 ± 8.1 | |||
| mTBI+, R mutation | 70.6 ± 11.9 | 58.8 ± 12.6 | 41.2 ± 12.4 | |||
| . | Major infection . | Acute GVHD . | Grades II-IV acute GVHD . | |||
|---|---|---|---|---|---|---|
| CIF plus or minus SE . | P . | CIF plus or minus SE . | P . | CIF plus or minus SE . | P . | |
| Interaction of mTBI and donor MBL2 mutation | .003 | .750 | .916 | |||
| mTBI−, D MBL2 wt | 32.1 ± 6.3 | 47.1 ± 6.8 | 30.8 ± 6.3 | |||
| mTBI−, D mutation | 40.6 ± 8.9 | 59.4 ± 8.9 | 31.3 ± 8.4 | |||
| mTBI+, D MBL2 wt | 50.8 ± 9.8 | 50.0 ± 9.7 | 35.7 ± 9.3 | |||
| mTBI+, D mutation | 76.2 ± 10.0 | 47.6 ± 11.3 | 28.6 ± 10.2 | |||
| Interaction of mTBI and recipient MBL2 mutation | .013 | .407 | .636 | |||
| mTBI−, R MBL2 wt | 31.1 ± 6.0 | 49.9 ± 6.5 | 31.6 ± 6.1 | |||
| mTBI−, R mutation | 44.4 ± 9.8 | 59.3 ± 9.8 | 29.6 ± 9.0 | |||
| mTBI+, R MBL2 wt | 57.2 ± 9.2 | 43.8 ± 9.0 | 28.1 ± 8.1 | |||
| mTBI+, R mutation | 70.6 ± 11.9 | 58.8 ± 12.6 | 41.2 ± 12.4 | |||
CIF indicates cumulative incidence fraction; SE, standard error; mTBI, myeloablative TBI; D, donor; wt, wild type; and R, recipient.
We next examined the effect of additional MBL2 variants on infection risk. The promoter haplotype LXA is associated with reduced MBL levels, and homozygosity for this haplotype, LXA/LXA, is associated with very low levels. However, MBL insufficiency, as defined as either LXA homozygosity or the presence of coding mutations, was a weaker risk factor for infection than MBL2 coding mutations alone (P = .24 for recipient genotypes; P = .110 for donor genotypes). Similarly, exclusion of HYA/D or LYA/D genotypes (which are associated with higher baseline MBL levels than the B and C coding mutations) from this genotypic definition of MBL sufficiency did not increase the strength of association with infection. We did not observe a significant association with either recipient or donor carriage of HYA, or the HYA/A genotypes and risk of infection.
MBL levels, as measured by either mannan-binding or C4-deposition assays, showed trends to association with infection, particularly with recipient levels. A baseline recipient mannan-binding level of less than 0.3 μg/mL (P = .174) and baseline C4-deposition level of less than 0.2 U/mL were weakly associated with infection (P = .148). Significant associations were not observed for levels measured at later time points, or with the absolute or relative increase in MBL levels from baseline to day 14 after transplantation.
In our previous retrospective study, we observed that the presence of donor MBL2 coding mutations were independently associated with risk of major infection following neutrophil count recovery, suggesting that donor-derived cells may synthesize clinically significant amounts of MBL after transplantation. Accordingly, we analyzed associations with infection both before and after neutrophil count recovery in the current study. In univariate analysis, both recipient and donor coding mutations were associated with infection after engraftment (Table 5).
MBL associations with infection occurring before and after neutrophil count recovery
| . | Major infection prior to neutrophil recovery . | Major infection after neutrophil recovery . | ||
|---|---|---|---|---|
| CIF plus or minus SE . | P . | CIF plus or minus SE . | P . | |
| Recipient mutation | .967 | .008 | ||
| No mutation | 26.8 ± 4.7 | 29.4 ± 7.9 | ||
| Mutation | 26.3 ± 6.9 | 43.2 ± 7.6 | ||
| Recipient MBL2-insufficient genotypes | .665 | .521 | ||
| Sufficient | 28.1 ± 4.4 | 35.1 ± 6.3 | ||
| Insufficient | 20.0 ± 8.2 | 24.0 ± 8.7 | ||
| Donor mutation | .306 | .066 | ||
| No mutation | 24.0 ± 4.9 | 30.0 ± 8.5 | ||
| Mutation | 30.6 ± 6.5 | 38.7 ± 7.0 | ||
| Donor MBL2-insufficient genotypes | .761 | .113 | ||
| Sufficient | 27.9 ± 4.9 | 30.4 ± 7.5 | ||
| Insufficient | 23.9 ± 6.4 | 38.0 ± 7.5 | ||
| . | Major infection prior to neutrophil recovery . | Major infection after neutrophil recovery . | ||
|---|---|---|---|---|
| CIF plus or minus SE . | P . | CIF plus or minus SE . | P . | |
| Recipient mutation | .967 | .008 | ||
| No mutation | 26.8 ± 4.7 | 29.4 ± 7.9 | ||
| Mutation | 26.3 ± 6.9 | 43.2 ± 7.6 | ||
| Recipient MBL2-insufficient genotypes | .665 | .521 | ||
| Sufficient | 28.1 ± 4.4 | 35.1 ± 6.3 | ||
| Insufficient | 20.0 ± 8.2 | 24.0 ± 8.7 | ||
| Donor mutation | .306 | .066 | ||
| No mutation | 24.0 ± 4.9 | 30.0 ± 8.5 | ||
| Mutation | 30.6 ± 6.5 | 38.7 ± 7.0 | ||
| Donor MBL2-insufficient genotypes | .761 | .113 | ||
| Sufficient | 27.9 ± 4.9 | 30.4 ± 7.5 | ||
| Insufficient | 23.9 ± 6.4 | 38.0 ± 7.5 | ||
CIF indicates cumulative incidence fraction; SE, standard error.
In multivariable analysis using stepwise backward variable selection, age at transplantation (P = .014), mTBI (P = .001), the use of gut decontamination (P = .034), and the presence of donor MBL2 coding mutations (P = .030) were significantly associated with major infection (Table 6). Recipient MBL2 genotype, was associated with postengraftment major infection in multivariable analysis, with a trend to an association with mTBI (Table 7).
Factors independently associated with major infection in competing risk multivariable analysis
| Factor . | Coefficient . | SE . | P . |
|---|---|---|---|
| Age at transplantation | 0.029 | 0.012 | .014 |
| mTBI | 0.946 | 0.261 | .001 |
| Gut decontamination | −0.554 | 0.260 | .034 |
| Donor MBL2 mutation | 0.568 | 0.261 | .030 |
| Factor . | Coefficient . | SE . | P . |
|---|---|---|---|
| Age at transplantation | 0.029 | 0.012 | .014 |
| mTBI | 0.946 | 0.261 | .001 |
| Gut decontamination | −0.554 | 0.260 | .034 |
| Donor MBL2 mutation | 0.568 | 0.261 | .030 |
Factors independently associated with major infection following neutrophil count recovery in competing risk multivariable analysis
| Factor . | Coefficient . | SE . | P . |
|---|---|---|---|
| Recipient MBL2 mutation | 0.83 | 0.324 | .01 |
| mTBI | 0.48 | 0.324 | .13 |
| Factor . | Coefficient . | SE . | P . |
|---|---|---|---|
| Recipient MBL2 mutation | 0.83 | 0.324 | .01 |
| mTBI | 0.48 | 0.324 | .13 |
Associations with other outcomes
No associations between MBL status, either as MBL levels or MBL2 genotype, and duration of fever, duration of neutropenia, survival, or transplant-related mortality were detected. The carriage of recipient MBL2-insufficient genotypes was associated with an increased risk of acute GVHD (P = .046) and grades II to IV acute GVHD (P = .043; Table 3). Clinical factors significantly associated with an increased cumulative incidence of acute GVHD were the use of gut decontamination (P = .007) and bone marrow as stem cell source (P = .012; Table 3). However, as few transplantations used peripheral blood stem cells in this study (n = 8), this result should be interpreted with caution.
Discussion
MBL deficiency is common and predisposes to infection in multiple clinical settings, especially when immune responses are otherwise compromised. There is consequently considerable interest in MBL replacement therapy17 to ameliorate serious infection in individuals considered at highest risk, based on either measurement of MBL levels or MBL2 genotyping. Despite the aggressive use of antimicrobial agents, infection remains a major barrier to the success of allo-HCT. Our previous retrospective data in related, myeloablative HLA-matched transplantations identified strong associations between MBL2 genotype and infection, suggesting that MBL replacement therapy may be clinically useful in this setting.10
This prospective study has examined MBL status in both myeloablative and reduced-intensity sibling, HLA-matched transplantations, has confirmed the association between MBL status and risk of infection in the first 100 days after transplantation, and has shown that associations are restricted to certain transplantation scenarios, most notably mTBI-conditioned myeloablative transplantations. The interaction between mTBI and MBL2 genotype was striking, with more than 70% of recipients with both risk factors experiencing infection. The markedly elevated risk of infection associated with mTBI use is likely to result from mucosal breaches allowing microbial invasion, and the delayed immune reconstitution associated with mTBI.18,19 This is consistent with the observation that the majority of infective episodes were bacteremic episodes, which may have resulted from egress of pathogens from the gut across damaged mucosae to the circulation. Notably, mTBI resulted in significant increases in MBL levels during the peritransplantation period, but only in individuals lacking MBL2 coding mutations. Unsurprisingly, it is the individuals with MBL2 coding mutations who are unable to increase MBL levels in the face of TBI and thus show the greatest risk of infection.
We previously reported independent associations of donor MBL2 genotype with infection risk after myeloablative allo-HCT.10 MBL is primarily synthesized by the liver,20 and it would be expected that recipient MBL synthetic capacity will exert the greatest influence on MBL levels and MBL-mediated immunity. The association with donor MBL2 genotype suggested that donor-derived hematopoietic tissues and their progeny might contribute clinically significant quantities of MBL after transplantation. However, a recent study of a small number of MBL2-deficient allogeneic hematopoietic stem cell transplant recipients failed to observe correction of MBL levels after receiving a transplant from an MBL-sufficient donor,21 thus questioning the importance of donor-derived MBL synthesis after BMT. The current study has again observed associations of both recipient and donor MBL2 genotype with infection risk in univariable analysis. In multivariable analysis, donor MBL2 genotype was independently associated with infection at any time after transplantation, whereas recipient, but not donor MBL status (MBL2 coding genotype and baseline MBL C4-deposition levels) were independently associated with infection after neutrophil engraftment. These seemingly contradictory observations are most likely explained by the high degree of MBL2 genotype sharing between the sibling donors and recipients in the current study—70.6% of sibling pairs shared identical MBL2 coding genotypes. Consequently, although MBL status is an independent risk factor for infection after transplantation, it is not possible to definitively state that donor MBL2 genotype is of greater importance than recipient MBL status in the current study. If an independent donor effect is confirmed in larger series, this would suggest that any donor-derived influence on MBL status and resistance to infection occurs at a local tissue level, without a substantive increase in measurable, circulating MBL levels.
In summary, we have identified independent associations between MBL2 genotype, myeloablative TBI, the use of gut decontamination, and the risk of major infection following sibling allo-HCT. These data suggest that trials of MBL replacement therapy in specific allo-HCT settings are now warranted. The group at highest risk of infection includes patients who underwent TBI-conditioned myeloablative transplantation in which either donor or recipient harbors MBL2 mutations. It can also be argued that alternative, non–TBI-conditioning regimens should be considered in recipients at greatest risk of infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Rosemary Hoyt, Jennifer Muirhead, and Angela Bayley for data collection.
This work was supported by the Cooperative Research Centre for Vaccine Technology, Australia.
Authorship
Contribution: C.G.M., S.L.H., K.D., and P.G.B. designed the study; K.F.B., A.P.S., and J.S. contributed clinical samples and collected clinical data; S.L.H., M.M.D., and R.M. performed laboratory assays; S.D. collected clinical data and maintained clinical databases; U.H. and P.G.B. curated clinical data; C.G.M. performed statistical analyses; C.G.M. and P.G.B. wrote the paper; all authors reviewed and commented on the paper.
Conflict-of-interest disclosure: C.G.M. is on the scientific advisory board of, and has received an honorarium from, Enzon Inc, which holds the license for recombinant human mannose-binding lectin.
Correspondence: Charles G. Mullighan, St Jude Children's Research Hospital, 262 Danny Thomas Place, Mail Stop 342, Memphis, TN, 38105-3678; e-mail: charles.mullighan@stjude.org; or Peter G. Bardy, Department of Clinical Haematology and Oncology, The Queen Elizabeth Hospital, Woodville Road, Adelaide, SA, 5000, Australia; e-mail: peter.bardy@imvs.sa.gov.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal