Abstract
Multiple myeloma (MM) is a cancer of plasma cells (PCs) expressing immunoglobulin heavy chain (IgH) postswitch isotypes. The discovery of earlier stage cells related to postswitch PCs, called preswitch clonotypic IgM (cIgM) cells led to the hypothesis that cIgM cells may be MM progenitors, replenishing the tumor throughout malignancy. cIgM cells may do this by undergoing class switch recombination (CSR), a process detectable in postswitch PCs as multiple IgH switch junctions associated with a single clonotypic IgH V/D/J. We addressed this with a specific clonotypic-switch polymerase chain reaction (PCR), informative for 32 of 41 cases. Here we made 2 significant discoveries: (1) in all cases, we detected only a single clonotypic switch fragment that persists over time (1-7.6 years), and (2) we detected ongoing mutation upstream of the switch junction in 5 of 6 patients, often targeting the intronic enhancer, a key control region in IgH expression. The presence of a single, unchanging clonotypic switch junction suggests that cIgM cells are not MM-PC progenitors; rather, postswitch PCs arise from a single cIgM cell, and MM-PC progenitors reside in the postswitch population. Furthermore, mutations revealed here provide a new marker to identify MM-PC progenitors and aggressive clones that evolve throughout malignancy.
Introduction
Multiple myeloma (MM) is an incurable B-lineage cancer characterized by malignant plasma cells (PCs) in the bone marrow (BM). Although chemotherapy often reduces tumor burden, most MM patients eventually relapse and succumb to the disease.1 In MM, malignant PCs share an identical clonotypic immunoglobulin heavy chain (IgH) V/D/J rearrangement that is unique for each patient, and can be detected by polymerase chain reaction (PCR).2 DNA sequence analysis has revealed somatically hypermutated but clonally homogeneous V/D/J in MM, suggesting a post–germinal center origin.3-5 Clonotypic V/D/J remains unchanged in MM-PCs throughout the course of disease, providing a stable tumor-specific marker.6,7 Previous studies suggest that relapse involves drug-resistant MM cells with progenitor-like or stemlike properties.8-12 The identity of these cells remains undetermined, although several studies have identified clonotypic B cells as possible MM stem cell candidates.7-19 Most MM patients have tumor PCs expressing clonotypic V/D/J in association with postswitch IgH constant region isotypes, either IgG or IgA.20 Interestingly, we and others have shown that in postswitch MM, coexisting clonotypic preswitch IgM (cIgM) cells are detectable in BM, blood, and mobilized blood autografts.14,15,21-23 The V/D/J of cIgM has a mutation profile identical to that of its postswitch tumor counterpart, and shows similar intraclonal homogeneity,14,15,23 suggesting that cIgM cells may have a malignant phenotype. The presence of the full spectrum of clonotypic clinical and nonclinical isotypes (eg, cIgM, IgD, and IgA in an MM patient of IgG clinical isotype) in the majority of MM patients also suggests that CSR has occurred in cIgM cells, and may be ongoing.22 Furthermore, work from our group demonstrates (1) that persistence of cIgM cells correlates with poor outcome and (2) cIgM cells show engraftment potential in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. Taken together, we have interpreted these studies to suggest a clinically relevant role for cIgM cells in MM.22 The characteristics of these cells remain unclear, in part because of the low frequency of cIgM cells in MM patients.22,23 Nevertheless, the consistent detection of cIgM in MM patients in several studies14,15,21-24 suggested that cIgM cells, however low in frequency, may be a more prevalent feature of postswitch MM than previously thought. In this study, we asked whether cIgM cells may serve as a progenitor pool for postswitch MM-PCs.
To assess the potential of cIgM cells as MM-PC progenitors, we examined clonotypic switch junctions in the postswitch MM-PCs. Normally, IgM cells undergo CSR via recombination between the IgM Sμ switch region and a downstream switch region, such as Sγ in the switch to IgG production (reviewed in Manis et al25 ). This process generates a hybrid Sμ/Sγ switch region with a unique junction. In cIgM cells undergoing multiple CSR events, as might be expected if they are progenitors for MM-PCs, multiple clonotypic switch junctions, or changes in the switch junction, are expected in the postswitch progeny. We addressed this possibility using a clonotypic switch PCR (CS-PCR), which specifically targets postswitch tumor cells by amplifying a DNA segment from the clonotypic V/D/J region to the end of the hybrid switch region. This effectively captures the hybrid switch junction, and allows us, by DNA fragment size analysis, to determine whether changes in clonotypic switch junctions have occurred. We conducted CS-PCR on MM patient samples, comparing the size of CS-PCR products amplified in paired samples taken at different time points. In addition, we sequenced the switch junctions of these amplified products to reveal any subtle changes not detectable by fragment size analysis. Finally, we expanded our sequence analysis to include the region upstream of the switch junction, including the CDR3 portion of the rearranged V/D/J gene, to determine whether any mutations had occurred over time. Using this approach, we determined that postswitch MM-PCs most likely originate from a single CSR event in a cIgM cell, and that cIgM cells do not play a direct role in generating postswitch MM-PCs. In addition, we identified ongoing mutation in postswitch MM-PCs that may provide a new marker for identifying potential MM-PC progenitor populations.
Methods
Patients
BM and blood (BL) samples were obtained from 40 MM and 1 monoclonal gammopathy of undetermined significance (MGUS) patient, after informed consent, at the Cross Cancer Institute, whose Institutional Review Board committee approved the study in compliance with the most recently revised Helsinki protocol. MM and MGUS were diagnosed according to Durie-Salmon staging.26 Diagnosis BM samples were taken on the first or second patient visit, before initiation of chemotherapy; relapse samples were taken approximately 1 to more than 7 years later. In cases where BM samples were not available, BL samples were used. International uniform response criteria were used to indicate the extent to which the MM clone was reduced by treatment.27
DNA, RNA extraction, and reverse transcription
Total RNA or DNA was isolated from density gradient purified patient peripheral blood or BM mononuclear cells (ranging from 1 to 10 × 106 cells per sample) using Trizol or DNAzol, respectively, according to the manufacturer's protocol (Invitrogen Life Technologies, Carlsbad, CA). Poly-A–tailed RNA from 1 μg total RNA was reverse transcribed for 1 hour at 42°C with 500 μM dT15, 500 μM of each dNTP, 10 mM dithiothreitol (DTT), 50 mM Tris-HCl, 75 mM KCl, 3 mM MgCl2, and 200 U Superscript Reverse Transcriptase (Invitrogen Life Technologies) in a 20-μL reaction, then inactivated at 99°C for 3 minutes. Bulk DNA samples were used in CS-PCR amplification of clonotypic V/D/J-S fragments. Bulk RNA samples were used in reverse-transcription (RT)–PCR detection of clonotypic V/D/J and cIgM transcripts.
Determination and detection of clonotypic IgH V/D/J and cIgM in MM patients
Clonotypic IgH V/D/J for each patient was identified by RT-PCR amplification of transcripts from bulk BM RNA using VH family consensus and constant region primers, followed by direct sequencing of the RT-PCR products. V/D/J sequences identified by this method were validated by single-cell RT-PCR using patient-specific CDR2 and CDR3 primers and cDNA from single CD138+ PCs sorted by flow cytometry.28 Proposed V/D/J sequences were accepted as clonotypic when present in the majority (> 75%) of individually tested single MM-PCs.7,28 Patient time point samples were assayed for clonotypic V/D/J either by single-stage PCR using patient-specific CDR2 and CDR3 sequences, or by nested PCR using VH family and JH consensus primers in the first stage, and CDR2 and CDR3 primers in the second stage. cIgM was detected by PCR as described in Reiman et al,22 using CDR3 sense and Cμ antisense primers.
CS-PCR
The DNA segment from the clonotypic V/D/J region to the end of the hybrid switch region (hereafter designated the V/D/J-S region) of MM patients was amplified in a 50-μL clonotypic switch (CS)–PCR reaction containing 10 to 200 ng bulk DNA as template, 60 mM Tris-SO4 (pH 8.9), 18 mM (NH4)2 SO4, 2.0 mM MgSO4, 200 μM of each dNTP, 200 nM primers (Table 1), 5 U Platinum Taq High Fidelity (Invitrogen Life Technologies), and 0% to 5% DMSO under the following cycling conditions: 94°C for 45 seconds; followed by 35 cycles of 94°C for 15 seconds, 57°C for 20 seconds, and 68°C for 6 minutes; followed by 68°C for 6 minutes. In cases where a nested PCR was necessary, 1 μL first-stage product was used in a second-stage reaction under identical conditions. A 1 to 10 μL aliquot of each amplification reaction was resolved by agarose gel electrophoresis and visualized under UV illumination. With regard to switch junction and mutation analysis, PCR products from 2 distinct reactions per patient sample time point were compared to identify disease-relevant mutations (common to both time point duplicates) and mutations due to Taq error (present in only one of the time point duplicates). The error rate of high-fidelity Taq in this study is calculated to be 1.7 × 10−4, close to our previously reported values of 1.3 × 10−4 bp using these reagents.23
PCR primers used in this study
| Primer* . | Nucleotide sequence . |
|---|---|
| Switch region | |
| Sγ1 3′ | TCCCAGTGTCCTGCATTACTTC |
| Sα1 3′ | AACCCAGCACAGAGAGGCCT |
| Constant region | |
| CγB | GGGGAAGACCGATGGGCCCT |
| CαB | GGGATTCGTGTAGTGCTTCACGTG |
| CS-PCR sequencing | |
| Sw1as | CCGTGTCCCAAGGTCAT |
| Sw1s | ATGACCTTGGGACACGG |
| Sw2as | GTGCTGCTGGGCTCAG |
| Sw2s | CTGAGCCCAGCAGCAC |
| Sw3as | CCTCCAAGACTCTGACCC |
| Sw3s | GGGTCAGAGTCTTGGAGG |
| Sw4as | CCTCAGAAGCCAGACCACT |
| Sw4s | AGTGGTCTGGCTTCTGAGG |
| Sw5as | CCAACCTGCAATGCTC |
| Sw5s | GAGCATTGCAGGTTGG |
| Sw6as | GCGCACCTCATAATTCTAAAG |
| Sw6s | CTTTAGAATTATGAGGTGCGC |
| Sw7as | TTAACAGTAAGCATGCCT |
| Sw7s | AGGCATGCTTACTGTTAA |
| Sw8as | CACCTGCTGTGGGTGC |
| Sw8s | GCACCCACAGCAGGTG |
| Sw9as | TGCTCAGTAGAGCACTTTCAG |
| Sw9s | CTGAAAGTGCTCTACTGAGCA |
| Sw10as | TGAAGGCCACTCTATCAGAA |
| Sw10s | TTCTGATAGAGTGGCCTTCA |
| Sw11as | TCCTGTCTTCAGCACTATCTGA |
| Sw11s | TCAGATAGTGCTGAAGACAGGA |
| Sw12as | GTCACTGCGAGCCTTGC |
| Sw12s | GCAAGGCTCGCAGTGAC |
| Sw13as | GTGCGATGATGACCTACAGTC |
| Sw13s | GACTGTAGGTCATCATCGCAC |
| Sw14as | TGTCTCCCTCTACTTGATACCC |
| Sw14s | GGGTATCAAGTAGAGGGAGACA |
| Sw15as | GAAAGCTGGATGGAGTTGTC |
| Sw15s | GACAACTCCATCCAGCTTTC |
| Primer* . | Nucleotide sequence . |
|---|---|
| Switch region | |
| Sγ1 3′ | TCCCAGTGTCCTGCATTACTTC |
| Sα1 3′ | AACCCAGCACAGAGAGGCCT |
| Constant region | |
| CγB | GGGGAAGACCGATGGGCCCT |
| CαB | GGGATTCGTGTAGTGCTTCACGTG |
| CS-PCR sequencing | |
| Sw1as | CCGTGTCCCAAGGTCAT |
| Sw1s | ATGACCTTGGGACACGG |
| Sw2as | GTGCTGCTGGGCTCAG |
| Sw2s | CTGAGCCCAGCAGCAC |
| Sw3as | CCTCCAAGACTCTGACCC |
| Sw3s | GGGTCAGAGTCTTGGAGG |
| Sw4as | CCTCAGAAGCCAGACCACT |
| Sw4s | AGTGGTCTGGCTTCTGAGG |
| Sw5as | CCAACCTGCAATGCTC |
| Sw5s | GAGCATTGCAGGTTGG |
| Sw6as | GCGCACCTCATAATTCTAAAG |
| Sw6s | CTTTAGAATTATGAGGTGCGC |
| Sw7as | TTAACAGTAAGCATGCCT |
| Sw7s | AGGCATGCTTACTGTTAA |
| Sw8as | CACCTGCTGTGGGTGC |
| Sw8s | GCACCCACAGCAGGTG |
| Sw9as | TGCTCAGTAGAGCACTTTCAG |
| Sw9s | CTGAAAGTGCTCTACTGAGCA |
| Sw10as | TGAAGGCCACTCTATCAGAA |
| Sw10s | TTCTGATAGAGTGGCCTTCA |
| Sw11as | TCCTGTCTTCAGCACTATCTGA |
| Sw11s | TCAGATAGTGCTGAAGACAGGA |
| Sw12as | GTCACTGCGAGCCTTGC |
| Sw12s | GCAAGGCTCGCAGTGAC |
| Sw13as | GTGCGATGATGACCTACAGTC |
| Sw13s | GACTGTAGGTCATCATCGCAC |
| Sw14as | TGTCTCCCTCTACTTGATACCC |
| Sw14s | GGGTATCAAGTAGAGGGAGACA |
| Sw15as | GAAAGCTGGATGGAGTTGTC |
| Sw15s | GACAACTCCATCCAGCTTTC |
Individual MM patient CDR2 and CDR3 primers have not been included in this list.
Sensitivity assay
A dilution series of an MM cell line in normal blood was created using LP-1 (IgG) cells aliquoted by flow cytometry and mixed with Ficoll purified normal blood cells. DNA from these cell mixtures was extracted using Trizol in the same manner as the MM BM and blood samples described in “DNA, RNA extraction, and reverse transcription.” CS-PCR reactions were conducted as mentioned in “CS-PCR,” with LP-1 CDR2-specific 5′ primers and the 3′ CγB constant region primer. The last dilution tube showing a distinguishable V/D/J-S product was used to calculate the sensitivity of the CS-PCR reaction.
Sequencing of CS-PCR products and switch junction determination
CS-PCR products were either (1) cloned and then sequenced, or (2) sequenced directly from CS-PCR reactions. In the first case, CS-PCR products were cloned using pCR4 TOPO TA vector and DH5alpha competent cells (Invitrogen Life Technologies), and plasmid miniprep DNA served as the sequencing template. In the second case, CS-PCR products were directly treated with ExoSapIt (USB, Cleveland, OH) then used as sequencing templates. In MM patients 1 and 2, no differences were observed in the switch junction sequences obtained from cloned or direct PCR products, suggesting that these methods are equivalent. In MM patients, 3 to 6 sequences were obtained directly from CS-PCR reactions. The V/D/J-S region amplified by CS-PCR was sequenced using sense and antisense Sw primers spaced approximately 300 bp apart, providing coverage of this region for both strands (Table 1; Sw15as has been previously described in Kriangkum et al29 ). Sequencing was performed with Big-Dye fluorescent sequencing kits and analyzed on an ABI 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA). V/D/J-S sequences were aligned and analyzed using Seqscape v2.1 (Applied Biosystems). The human chromosome 14 genomic sequence NT 026437.11 from bases 87332713 (JH1) to 87324286 (end of Sμ) was used as a reference to align CS-PCR sequences. Switch junctions identified in Seqscape were further characterized using BLAST30,31 (low-complexity filter disabled) to identify Sμ and Sγ sequences and ClustalW32,33 to refine switch junction alignments. Switch junction numbering was assigned using position 87327671 of NT 026437.11, corresponding to the start of the Sμ pentameric repeat region first described by Mills et al.34 Numbering of the Sγ1, 2, and 3 junctions was determined by BLAST and ClustalW using GenBank accession numbers U39737, U39934, and U39935.1, respectively.35,36 In the mutation analysis, new mutations were defined as mutations present in the second time point sample that were not observed in the first time point sample. New mutations were considered valid if they appeared in both of the time point duplicates originating from distinct PCR reactions, to distinguish new mutations from misincorporated bases due to Taq error.
Results
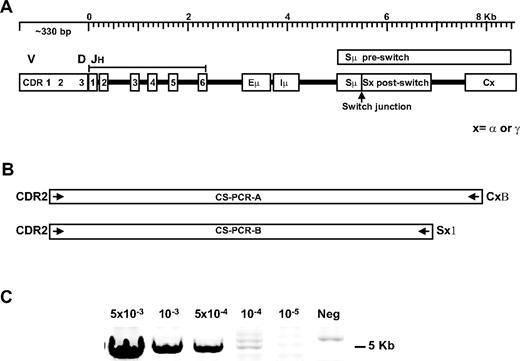
DNA sequences encompassing the rearranged V/D/J and downstream hybrid switch region DNA are amplified by a clonotypic switch PCR
cIgM is present in the majority of postswitch (IgG or IgA) patients.22 If these cells undergo CSR during the course of disease in MM patients, the CSR junction, which is a clonotypic marker, is predicted to differ at the times of diagnosis and relapse. We used long-distance CS-PCR to capture this information (Figure 1A). The CS-PCR includes patient-specific CDR2 primers and CSR-specific switch or constant region primers to selectively amplify clonotypic switch fragments (Figure 1B; Table 1). The amplified region includes several key elements: (1) the rearranged Ig heavy chain VH gene downstream of CDR2, including the rearranged DH and JH, (2) the remaining unrearranged JH genes, (3) the Eμ intronic IgH enhancer controlling immunoglobulin transcription,37 (4) the Iμ exon where sterile transcripts in CSR are produced,38 and (5) the Sμ switch region, the Sμ/Sγ (or Sα) switch junction, and a portion of the downstream Sγ (or Sα) switch region.34 CS-PCR products range from 3.5 to 9 Kb for the patients in this study, varying due to JH use and different hybrid switch region lengths. The majority of MM patient clonotypic switch fragments could be captured by this method. For patient samples where CS-PCR failed initially, a positive result was obtained by substituting the downstream switch region primer with the constant region primer, suggesting that base changes in the switch primer target sequence are the most likely cause of CS-PCR failure. The CS-PCR reaction can detect as little as 1 CSR target in 104 cells in the single-stage reaction, as judged by limiting dilution analysis of an MM control cell line into normal blood (Figure 1C). In samples with a significant tumor burden, the CS-PCR required very little optimization; in other patients with a presumptively lower tumor burden, it was necessary to titrate DNA template concentration, Mg2+, and in some cases, to include DMSO to amplify the V/D/J-S region. In samples where the tumor burden is quite low, a nested PCR was used (PCR A as first stage, and PCR B as second stage, described in Table 2 in the CS-PCR column as “AB”).
Clonotypic switch (CS-PCR) amplification in MM patients. (A) A diagram of the rearranged immunoglobulin heavy chain locus: V indicates variable; D, diversity; JH, junction segments; Eμ, intronic enhancer; Iμ, I exon; Sμ, switch region before class switching; Sμ/Sx, hybrid switch region generated after class switching; C, downstream constant region; and x, α (IgA) or γ (IgG) isotope regions. (B) CS-PCR-A and CS-PCR-B, with patient-specific CDR2 and CSR-specific Sx1 or CxB primers to enable selective amplification of clonotypic switch products. For some cases, CS-PCR-A products were used in a nested CS-PCR-B reaction, called CS-PCR-AB, to increase sensitivity. (C) Sensitivity assay for the CS-PCR-A reaction using LP1 cells diluted into normal blood. Cell concentration is shown above the panel; neg indicates no LP1 cells added.
Clonotypic switch (CS-PCR) amplification in MM patients. (A) A diagram of the rearranged immunoglobulin heavy chain locus: V indicates variable; D, diversity; JH, junction segments; Eμ, intronic enhancer; Iμ, I exon; Sμ, switch region before class switching; Sμ/Sx, hybrid switch region generated after class switching; C, downstream constant region; and x, α (IgA) or γ (IgG) isotope regions. (B) CS-PCR-A and CS-PCR-B, with patient-specific CDR2 and CSR-specific Sx1 or CxB primers to enable selective amplification of clonotypic switch products. For some cases, CS-PCR-A products were used in a nested CS-PCR-B reaction, called CS-PCR-AB, to increase sensitivity. (C) Sensitivity assay for the CS-PCR-A reaction using LP1 cells diluted into normal blood. Cell concentration is shown above the panel; neg indicates no LP1 cells added.
CS-PCR fragment analysis in paired MM time point samples
| Patient no.*/ Sample† . | Isotype . | Time between samples, y . | Transplantation status‡ . | Treatment response§ . | CS-PCR product . | VDJ-PCR# . | cIgM** . | |
|---|---|---|---|---|---|---|---|---|
| Length‖ . | PCR type¶ . | |||||||
| Patient 1 | ||||||||
| BM1 | IgG | 4.2 | ASCT | VGPR | 5389 | B | + | + |
| BM2 | IgG | 5389 | B | + | + | |||
| Patient 2 | ||||||||
| BM1 | IgG | 1.6 | nt | PR | 7114 | B | + | + |
| BM2R | IgG | 7114 | B | + | + | |||
| Patient 3 | ||||||||
| BM1 | IgG | 2.3 | ASCT | SD | 5296 | B | + | − |
| BM3R | IgG | 5296 | B | + | − | |||
| Patient 4 | ||||||||
| BM1 | IgG | 1.6 | ASCT | VGPR | 5914 | B | + | + |
| BL2 | IgG | 5914 | B | + | + | |||
| Patient 5 | ||||||||
| BL1 | IgG | 2.3 | nt | PR | 4565 | B | + | + |
| BM2R | IgG | 4565 | B | + | + | |||
| Patient 6 | ||||||||
| BM1 | IgG | 1.8 | ASCT | PR | 6442 | B | + | − |
| BM2R | IgG | 6442 | B | + | + | |||
| Patient 7 | ||||||||
| BL1 | IgG | 2.8 | ASCT | VGPR | 6500 | AB | + | nd |
| BL2R | IgG | 6500 | AB | + | nd | |||
| Patient 8 | ||||||||
| BM1 | IgA | 1.4 | nt | SD | 5500 | B | + | nd |
| BM2 | IgA | 5500 | B | + | nd | |||
| Patient 9 | ||||||||
| BM1 | IgG | 6.4 | nt | PR | 6000 | AB | + | − |
| BL2 | IgG | 6000 | AB | + | + | |||
| Patient 10 | ||||||||
| BL1 | IgG | 1.8 | nt | PR | 6000 | A | + | + |
| BL2 | IgG | 6000 | A | + | + | |||
| Patient 11 | ||||||||
| BM1 | IgG | 1.7 | nt | SD | 5000 | A | + | nd |
| BM2 | IgG | 5000 | A | + | nd | |||
| Patient 12 | ||||||||
| BM1 | IgG | 1.9 | ALLO | VGPR | 5000 | B | + | nd |
| BM2 | IgG | 5000 | B | + | nd | |||
| Patient 13 | ||||||||
| BL1 | IgA | 2.1 | ASCT | VGPR | 6500 | B | + | − |
| BL2 | IgA | 6500 | B | + | − | |||
| Patient 14 | ||||||||
| BM1R | IgG | 4.7 | ASCT | VGPR | 7500 | A | + | nd |
| BM2 | IgG | 7500 | A | + | nd | |||
| Patient 15 | ||||||||
| BM1 | IgG | 4.8 | ASCT | CR | 8000 | A | + | nd |
| BM2 | IgG | 8000 | A | + | nd | |||
| Patient 16 | ||||||||
| BM1 | IgG | 1.2 | nt | PR | 6000 | B | + | nd |
| BM2 | IgG | 6000 | B | + | nd | |||
| Patient 17 | ||||||||
| BM1 | IgG | 2.3 | nt | PD | 4500 | B | + | nd |
| BM2 | IgG | 4500 | B | + | nd | |||
| Patient 18 | ||||||||
| BL1 | IgG | 6.6 | nt | PR | 3500 | A | + | + |
| BL2 | IgG | 3500 | A | + | + | |||
| Patient 19 | ||||||||
| BM1 | IgG | 1.4 | ASCT | PR | 7000 | A | + | + |
| BM2R | IgG | 7000 | A | + | + | |||
| Patient 20 | ||||||||
| BM1 | IgG | 1.4 | nt | PR | 6000 | B | + | + |
| BM2 | IgG | 6000 | B | + | + | |||
| Patient 21 | ||||||||
| BL1 | IgA | 1.3 | ASCT | VGPR | 8000 | A | + | + |
| BL2 | IgA | 8000 | A | + | + | |||
| Patient 22 | ||||||||
| BM1 | IgA | 1.9 | ASCT | PR | 5000 | B | + | nd |
| BM2R | IgA | 5000 | B | + | nd | |||
| Patient 23 | ||||||||
| BL1 | IgG | 7.6 | ASCT | VGPR | 4500 | B | + | + |
| BL2 | IgG | 4500 | B | + | + | |||
| Patient 24 | ||||||||
| BM1 | IgG | 5.0 | nt | PR | 5000 | B | + | + |
| BM2R | IgG | 5000 | B | + | + | |||
| Patient 25 | ||||||||
| BL1 | IgG | 1.5 | ASCT | PR | 5000 | A | + | nd |
| BL2 | IgG | 5000 | A | + | nd | |||
| Patient 26 | ||||||||
| BM1 | IgG | 2.4 | nt | VGPR | 6000 | B | + | nd |
| BM2 | IgG | 6000 | B | + | nd | |||
| Patient 27 | ||||||||
| BL1 | IgG | 2.0 | nt | PR | 5000 | B | + | + |
| BL2 | IgG | 5000 | B | + | + | |||
| Patient 28 | ||||||||
| BM1 | IgG | 5.0 | ASCT | VGPR | 5500 | AB | + | + |
| BL2 | IgG | 5500 | AB | + | + | |||
| Patient 29 | ||||||||
| BL1 | IgA | 1.9 | ASCT | PR | 3500 | A | + | + |
| BL2 | IgA | 3500 | A | + | − | |||
| Patient 30 | ||||||||
| BM1 | IgG | 1.0 | nt | SD | 6000 | A | + | nd |
| BM2 | IgG | 5000 | AB | + | nd | |||
| Patient 31 | ||||||||
| BM1 | IgA | 7.5 | ASCT | VGPR | 5500 | A | + | + |
| BL2 | IgA | 5500 | A | + | + | |||
| Patient 32 | ||||||||
| BL1 | IgA | 2.9 | ASCT | VGPR | 5000 | A | + | − |
| BL2 | IgA | 5000 | A | + | − | |||
| Patient 33 | ||||||||
| BM1 | IgG | 1.0 | ASCT | VGPR | 5500 | A | + | nd |
| BL2 | IgG | - | AB | + | nd | |||
| Patient 34 | ||||||||
| BM1 | IgA | 6.7 | nt | PR | 5900 | A | + | + |
| BL2 | IgA | - | AB | + | + | |||
| Patient 35 | ||||||||
| BM1 | IgG | 1.0 | ASCT | PR | 6500 | A | + | nd |
| BM2 | IgG | - | AB | - | nd | |||
| Patient 36 | ||||||||
| BL1 | IgG | 4.4 | ASCT | VGPR | 4500 | A | + | − |
| BL2 | IgG | - | AB | − | − | |||
| Patient 37 | ||||||||
| BM1 | IgG | 3.2 | ASCT | PR | 6000 | A | + | nd |
| BM2 | IgG | - | AB | − | nd | |||
| Patient 38 | ||||||||
| BM1 | IgG | 2.0 | ASCT | PR | 9000 | A | + | nd |
| BL2 | IgG | − | AB | − | nd | |||
| Patient 39 | ||||||||
| BM1 | IgG | 1.5 | ASCT | VGPR | − | AB | + | nd |
| BM2 | IgG | − | AB | + | nd | |||
| Patient 40 | ||||||||
| BM1 | IgA | 1.9 | ASCT | PR | − | AB | + | nd |
| BM2 | IgA | − | AB | + | nd | |||
| Patient 41 | ||||||||
| BM1 | IgG | 1.0 | nt | PR | − | AB | − | nd |
| BM2 | IgG | − | AB | + | nd | |||
| Patient no.*/ Sample† . | Isotype . | Time between samples, y . | Transplantation status‡ . | Treatment response§ . | CS-PCR product . | VDJ-PCR# . | cIgM** . | |
|---|---|---|---|---|---|---|---|---|
| Length‖ . | PCR type¶ . | |||||||
| Patient 1 | ||||||||
| BM1 | IgG | 4.2 | ASCT | VGPR | 5389 | B | + | + |
| BM2 | IgG | 5389 | B | + | + | |||
| Patient 2 | ||||||||
| BM1 | IgG | 1.6 | nt | PR | 7114 | B | + | + |
| BM2R | IgG | 7114 | B | + | + | |||
| Patient 3 | ||||||||
| BM1 | IgG | 2.3 | ASCT | SD | 5296 | B | + | − |
| BM3R | IgG | 5296 | B | + | − | |||
| Patient 4 | ||||||||
| BM1 | IgG | 1.6 | ASCT | VGPR | 5914 | B | + | + |
| BL2 | IgG | 5914 | B | + | + | |||
| Patient 5 | ||||||||
| BL1 | IgG | 2.3 | nt | PR | 4565 | B | + | + |
| BM2R | IgG | 4565 | B | + | + | |||
| Patient 6 | ||||||||
| BM1 | IgG | 1.8 | ASCT | PR | 6442 | B | + | − |
| BM2R | IgG | 6442 | B | + | + | |||
| Patient 7 | ||||||||
| BL1 | IgG | 2.8 | ASCT | VGPR | 6500 | AB | + | nd |
| BL2R | IgG | 6500 | AB | + | nd | |||
| Patient 8 | ||||||||
| BM1 | IgA | 1.4 | nt | SD | 5500 | B | + | nd |
| BM2 | IgA | 5500 | B | + | nd | |||
| Patient 9 | ||||||||
| BM1 | IgG | 6.4 | nt | PR | 6000 | AB | + | − |
| BL2 | IgG | 6000 | AB | + | + | |||
| Patient 10 | ||||||||
| BL1 | IgG | 1.8 | nt | PR | 6000 | A | + | + |
| BL2 | IgG | 6000 | A | + | + | |||
| Patient 11 | ||||||||
| BM1 | IgG | 1.7 | nt | SD | 5000 | A | + | nd |
| BM2 | IgG | 5000 | A | + | nd | |||
| Patient 12 | ||||||||
| BM1 | IgG | 1.9 | ALLO | VGPR | 5000 | B | + | nd |
| BM2 | IgG | 5000 | B | + | nd | |||
| Patient 13 | ||||||||
| BL1 | IgA | 2.1 | ASCT | VGPR | 6500 | B | + | − |
| BL2 | IgA | 6500 | B | + | − | |||
| Patient 14 | ||||||||
| BM1R | IgG | 4.7 | ASCT | VGPR | 7500 | A | + | nd |
| BM2 | IgG | 7500 | A | + | nd | |||
| Patient 15 | ||||||||
| BM1 | IgG | 4.8 | ASCT | CR | 8000 | A | + | nd |
| BM2 | IgG | 8000 | A | + | nd | |||
| Patient 16 | ||||||||
| BM1 | IgG | 1.2 | nt | PR | 6000 | B | + | nd |
| BM2 | IgG | 6000 | B | + | nd | |||
| Patient 17 | ||||||||
| BM1 | IgG | 2.3 | nt | PD | 4500 | B | + | nd |
| BM2 | IgG | 4500 | B | + | nd | |||
| Patient 18 | ||||||||
| BL1 | IgG | 6.6 | nt | PR | 3500 | A | + | + |
| BL2 | IgG | 3500 | A | + | + | |||
| Patient 19 | ||||||||
| BM1 | IgG | 1.4 | ASCT | PR | 7000 | A | + | + |
| BM2R | IgG | 7000 | A | + | + | |||
| Patient 20 | ||||||||
| BM1 | IgG | 1.4 | nt | PR | 6000 | B | + | + |
| BM2 | IgG | 6000 | B | + | + | |||
| Patient 21 | ||||||||
| BL1 | IgA | 1.3 | ASCT | VGPR | 8000 | A | + | + |
| BL2 | IgA | 8000 | A | + | + | |||
| Patient 22 | ||||||||
| BM1 | IgA | 1.9 | ASCT | PR | 5000 | B | + | nd |
| BM2R | IgA | 5000 | B | + | nd | |||
| Patient 23 | ||||||||
| BL1 | IgG | 7.6 | ASCT | VGPR | 4500 | B | + | + |
| BL2 | IgG | 4500 | B | + | + | |||
| Patient 24 | ||||||||
| BM1 | IgG | 5.0 | nt | PR | 5000 | B | + | + |
| BM2R | IgG | 5000 | B | + | + | |||
| Patient 25 | ||||||||
| BL1 | IgG | 1.5 | ASCT | PR | 5000 | A | + | nd |
| BL2 | IgG | 5000 | A | + | nd | |||
| Patient 26 | ||||||||
| BM1 | IgG | 2.4 | nt | VGPR | 6000 | B | + | nd |
| BM2 | IgG | 6000 | B | + | nd | |||
| Patient 27 | ||||||||
| BL1 | IgG | 2.0 | nt | PR | 5000 | B | + | + |
| BL2 | IgG | 5000 | B | + | + | |||
| Patient 28 | ||||||||
| BM1 | IgG | 5.0 | ASCT | VGPR | 5500 | AB | + | + |
| BL2 | IgG | 5500 | AB | + | + | |||
| Patient 29 | ||||||||
| BL1 | IgA | 1.9 | ASCT | PR | 3500 | A | + | + |
| BL2 | IgA | 3500 | A | + | − | |||
| Patient 30 | ||||||||
| BM1 | IgG | 1.0 | nt | SD | 6000 | A | + | nd |
| BM2 | IgG | 5000 | AB | + | nd | |||
| Patient 31 | ||||||||
| BM1 | IgA | 7.5 | ASCT | VGPR | 5500 | A | + | + |
| BL2 | IgA | 5500 | A | + | + | |||
| Patient 32 | ||||||||
| BL1 | IgA | 2.9 | ASCT | VGPR | 5000 | A | + | − |
| BL2 | IgA | 5000 | A | + | − | |||
| Patient 33 | ||||||||
| BM1 | IgG | 1.0 | ASCT | VGPR | 5500 | A | + | nd |
| BL2 | IgG | - | AB | + | nd | |||
| Patient 34 | ||||||||
| BM1 | IgA | 6.7 | nt | PR | 5900 | A | + | + |
| BL2 | IgA | - | AB | + | + | |||
| Patient 35 | ||||||||
| BM1 | IgG | 1.0 | ASCT | PR | 6500 | A | + | nd |
| BM2 | IgG | - | AB | - | nd | |||
| Patient 36 | ||||||||
| BL1 | IgG | 4.4 | ASCT | VGPR | 4500 | A | + | − |
| BL2 | IgG | - | AB | − | − | |||
| Patient 37 | ||||||||
| BM1 | IgG | 3.2 | ASCT | PR | 6000 | A | + | nd |
| BM2 | IgG | - | AB | − | nd | |||
| Patient 38 | ||||||||
| BM1 | IgG | 2.0 | ASCT | PR | 9000 | A | + | nd |
| BL2 | IgG | − | AB | − | nd | |||
| Patient 39 | ||||||||
| BM1 | IgG | 1.5 | ASCT | VGPR | − | AB | + | nd |
| BM2 | IgG | − | AB | + | nd | |||
| Patient 40 | ||||||||
| BM1 | IgA | 1.9 | ASCT | PR | − | AB | + | nd |
| BM2 | IgA | − | AB | + | nd | |||
| Patient 41 | ||||||||
| BM1 | IgG | 1.0 | nt | PR | − | AB | − | nd |
| BM2 | IgG | − | AB | + | nd | |||
Patients 7, 12, 13, 16, 20, and 22 were diagnosed as MM stage II and progressed to MMIII by the second sample. In patient 17, BM1 was diagnosed as MGUS; BM2, as MM1. The remaining patients were MMIII.
Sample codes: BM indicates bone marrow; BL, blood; and R, relapse; numbers designate sample order.
Transplantation status during study interval: ASCT indicates autologous stem cell transplantation; ALLO, allogeneic stem cell transplantation; and nt, did not undergo transplantation.
Response to at least one of the treatments administered during the study interval was as follows: complete response, CR (myeloma clone is clinically undetectable) = 2.4%; very good partial response, VGPR (≥90% decrease in M-component) = 36.6%; partial response, PR (≥50% decrease in M-component) = 48.8%; progressive disease, PD (≥25% increase in M-component) = 2.4%; and stable disease, SD (not satisfying previously mentioned criteria) = 9.8%.
CS-PCR fragment lengths are given in base pairs; for the first 6 patients, switch junctions were identified by sequencing, enabling a more exact determination of the fragment length.
CS-PCR type A and B are single-stage reactions; AB is a nested PCR using A primers in the first stage and B primers in the second stage (Figure 1); + indicates a PCR product was detected; −, no product detected.
V/D/J-PCR indicates presence of MM tumor cells.
cIgM-PCR indicates presence of MM cells expressing clonotypic IgM; nd indicates not tested.
CS fragment sizes remain unchanged over time
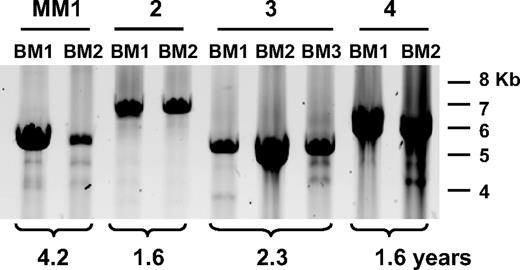
We conducted a CS-PCR analysis of 40 MM and 1 MGUS patient (Table 2). The majority of patients presented here have undergone therapy resulting in a significant reduction in tumor burden, followed by relapse. A smaller fraction of cases with stable or progressive disease have also been included. CS-PCR experiments were conducted with 2 samples per MM patient. For most patients these samples included diagnosis BM aspirates and samples taken at a subsequent time point, such as a relapse BM or a BL sample if no BM was available. We tested sets of samples taken from 1 to more than 7 years apart to enable accumulation of new clonotypic switch variants. Most patients underwent treatment during the period of study (39/41; 95%); more than half of these underwent either autologous or allogeneic stem cell transplantation (25/41; 61% [Table 2]). In the interval between the 2 time points, most patients showed a significant decrease in tumor burden as judged by international uniform response criteria (36/41; 88% [Table 2]).27 In this cohort, CS-PCR products were detected in 38 (93%) of 41 patients; in CS-PCR–positive patients, 32 (84%) of 38 were informative and positive for both time point samples. A V/D/J-PCR was included to monitor patient tumor burden and a cIgM-specific PCR, to monitor cIgM. In patients where a second time point sample was CS-PCR negative, the sample was also V/D/J negative, indicating that low tumor burden was responsible for CS-PCR negativity. Four (11%) of 38 CS-PCR–negative patients were V/D/J positive, suggesting that the CS-PCR is not as sensitive as the V/D/J-PCR, or that mutation has occurred in the downstream CS-PCR primer site. In 22 of 41 cases, cIgM-specific PCR was conducted, and the majority (86%) had detectable cIgM cells in one or more of the tested samples. In each informative case, CS-PCR products observed in the first and second time point samples were the same size (Table 2; Figure 2), with no indication that any new bands had been generated over time. This includes a case that converted from MGUS to MM (Table 2, patient 17). If cIgM cells do continue to generate postswitch PCs throughout disease, this activity must occur in less than 1 in 10 000 cells, below the sensitivity of the single-stage reaction. In several instances, extra bands in the appropriate size range were observed in addition to the main clonotypic band, but these bands were very faint and did not change in intensity in the second time point sample. Furthermore, in samples where it was necessary to use a nested PCR with higher sensitivity to amplify the V/D/J-S product, no other bands that could be ascribed to continuous switching of the cIgM population were consistently observed. Thus, observations from the CS-PCR analysis alone strongly suggest that class-switched PC progeny from a single IgM clone predominate throughout disease.
CS-PCR in MM patient time point samples. A representative CS-PCR-A in patients MM1 to MM4 is shown above the panel. BM indicates bone marrow; BL, blood. Time point number is given for each sample, and the time between samples is indicated below the panel. Molecular weight markers are shown on the right. Faint secondary bands below the strong CS-PCR band arise from nonspecific priming by the downstream Sγ1 primer.
CS-PCR in MM patient time point samples. A representative CS-PCR-A in patients MM1 to MM4 is shown above the panel. BM indicates bone marrow; BL, blood. Time point number is given for each sample, and the time between samples is indicated below the panel. Molecular weight markers are shown on the right. Faint secondary bands below the strong CS-PCR band arise from nonspecific priming by the downstream Sγ1 primer.
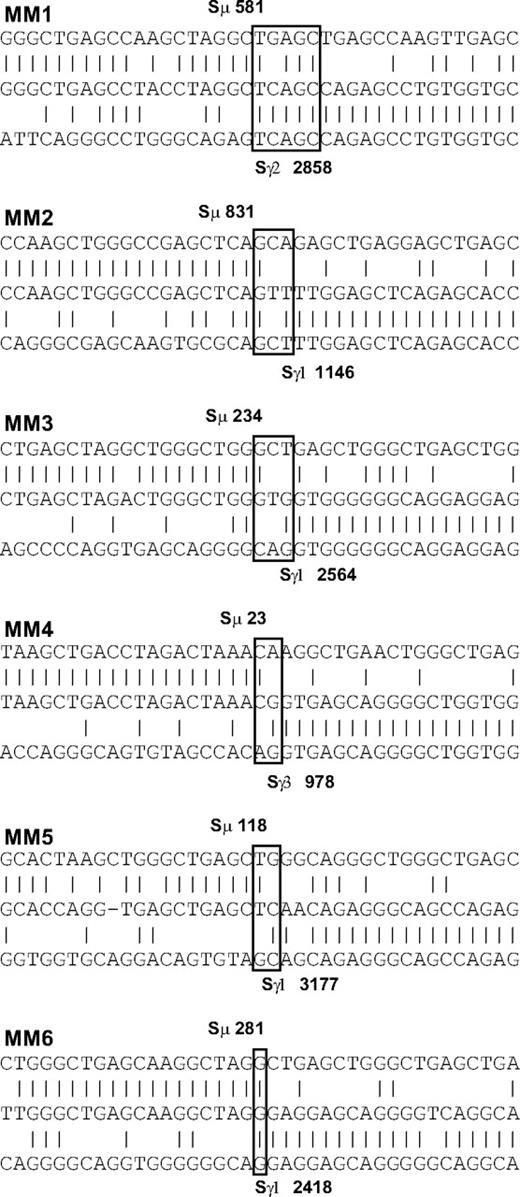
The clonotypic switch junction sequence does not change over time
Although the CS-PCR product sizes appear identical by gel electrophoresis, DNA sequence differences in the switch junction may remain undetected by this method. We addressed this issue by sequencing the switch junctions of randomly chosen MM patients for both sample time points. Because Sμ is GC rich and contains tandem repeats, this sequence cannot be used as a target region for sequencing primers. As a result, we obtained information only from samples where the switch junction was within the range of our sequencing primer upstream of Sμ. We identified switch junctions for 6 of 7 patients within approximately the first 1 Kb of the Sμ tandem repeats (Figure 3; Table 3). MM clonotypic switch junctions appeared normal, with one junction containing a short microhomology (patient MM1), reminiscent of longer microhomologies associated with deficiencies in genes of the CSR pathway.39,40 In all cases, only one clonotypic switch junction was identified per patient, and the switch junction remained constant throughout the course of disease, lasting up to 4 years in one patient. Of the 6 patients, 5 achieved either VGPR or PR during the interval studied (Table 2), suggestive of a reduced tumor burden, thereby providing an opportunity for expansion of any clones harboring new CSR events. This detailed switch junction sequence analysis strongly supports the CS-PCR results, suggesting that ongoing CSR does not occur at any significant level in MM.
Switch junction analysis in 6 MM patients. Homologies between switch regions and the switch junction sequence were determined by BLAST and ClustalW analysis. Switch junctions are indicated with open boxes. Upper sequence indicates Sμ switch region; center, switch junction sequence from this study; and lower, Sγ switch region. Switch base positions are shown in bold. Vertical lines indicate sequence identity; dashes, spaces introduced for optimal alignment. A switch junction microhomology of 4 to 5 bp is evident in patient MM1.
Switch junction analysis in 6 MM patients. Homologies between switch regions and the switch junction sequence were determined by BLAST and ClustalW analysis. Switch junctions are indicated with open boxes. Upper sequence indicates Sμ switch region; center, switch junction sequence from this study; and lower, Sγ switch region. Switch base positions are shown in bold. Vertical lines indicate sequence identity; dashes, spaces introduced for optimal alignment. A switch junction microhomology of 4 to 5 bp is evident in patient MM1.
Clonotypic switch junction and mutation analysis in MM patient V/D/J-S regions
| Patient* . | IgH germ-line genes . | Post switch constant region . | Elapsed time, y† . | Shared mutations, by region‡ . | New mutations§ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| JH region . | JH6-Enh . | Enh . | Enh-Sμ . | Total . | JH-Sμ . | Total . | ||||
| MM1 | VH2-70 DH3-9 JH3b | IgG2 | 4.2 | 71, 3Δ, 1 ins | 3 | 1 | 12, 4Δ | 95 | 7, 2Δ | 9 |
| MM2 | VH2-5 DH6-19 JH3b | IgG1 | 1.6 | 86, 3Δ, 1 ins | 13 | 2 | 18, 1 ins | 129 | 3 | 3 |
| MM3 | VH3-66 DH3-9 JH4b | IgG1 | 2.3 | 29, 2Δ | 4 | 1 | 5, 2Δ | 43 | 7, 1 td | 8 |
| MM4 | VH3-53 DH2-15 JH4b | IgG3 | 1.6 | 102, 2Δ, 1 ins | 9 | 1 | 12, 2Δ, 1 ins | 130 | 5, 1Δ | 6 |
| MM5 | VH3-36 DH6-19 JH4b | IgG1 | 2.3 | 45 | 4 | 0 | 3 | 53 | 3 | 3 |
| MM6 | VH3-30.3 JH3b‖ | IgG1 | 1.8 | 63, 1Δ, 2 ins | 7 | 1 | 3, 1 ins | 78 | 0 | 0 |
| Patient* . | IgH germ-line genes . | Post switch constant region . | Elapsed time, y† . | Shared mutations, by region‡ . | New mutations§ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| JH region . | JH6-Enh . | Enh . | Enh-Sμ . | Total . | JH-Sμ . | Total . | ||||
| MM1 | VH2-70 DH3-9 JH3b | IgG2 | 4.2 | 71, 3Δ, 1 ins | 3 | 1 | 12, 4Δ | 95 | 7, 2Δ | 9 |
| MM2 | VH2-5 DH6-19 JH3b | IgG1 | 1.6 | 86, 3Δ, 1 ins | 13 | 2 | 18, 1 ins | 129 | 3 | 3 |
| MM3 | VH3-66 DH3-9 JH4b | IgG1 | 2.3 | 29, 2Δ | 4 | 1 | 5, 2Δ | 43 | 7, 1 td | 8 |
| MM4 | VH3-53 DH2-15 JH4b | IgG3 | 1.6 | 102, 2Δ, 1 ins | 9 | 1 | 12, 2Δ, 1 ins | 130 | 5, 1Δ | 6 |
| MM5 | VH3-36 DH6-19 JH4b | IgG1 | 2.3 | 45 | 4 | 0 | 3 | 53 | 3 | 3 |
| MM6 | VH3-30.3 JH3b‖ | IgG1 | 1.8 | 63, 1Δ, 2 ins | 7 | 1 | 3, 1 ins | 78 | 0 | 0 |
GenBank accession numbers for patients MM1 to MM6 for time points 1 and 2, respectively, are as follows: MM1: EU687200, EU687201; MM2: EU687202, EU687203; MM3: EU687204, EU687205; MM4: EU687206, EU687207; MM5: EU687208, EU687209; and MM6: EU687210, EU687211.
The time elapsed between first and second time point samples.
Common mutations in first and second time point samples, compared with the IgH locus reference sequence NT 026437.11 bases 87332713 to 87324286. The number of point mutations are listed first; followed by Δ, deletions. ins indicates insertions; td, tandem duplication.
New mutations in the CS-PCR fragment of the second time point samples are listed: mutations are described as described earlier; td indicates tandem duplication.
A definitive D gene assignment could not be determined for MM6.
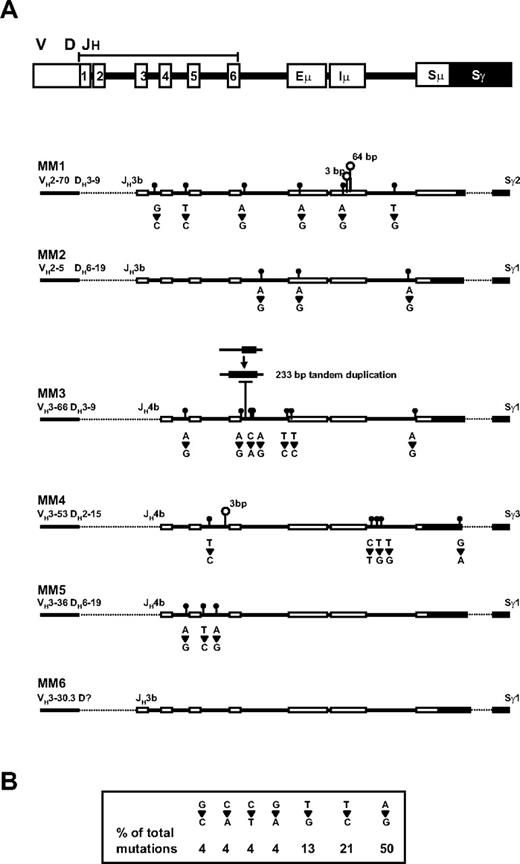
Ongoing mutation is detected in MM patient V/D/J-S regions
Whereas our analysis of CS-PCR products revealed no changes in size or switch junction sequence over time, sequence analysis of the V/D/J-S region upstream of the junction revealed a small increase in mutations in the later time point samples in 5 of 6 patients whose test data are summarized in Table 3 and Figure 4A. In this analysis, the V/D/J-S regions were sequenced for the first and second time points of each patient. Sw primers were used to cover this region; Sγ primers, to confirm the presence of an IgG switch; and CDR2/3 primers, to confirm that the V/D/J sequence was clonotypic. Full sequence coverage, with very few gaps (see Table 3 for GenBank accession numbers) was obtained for the JH region through to the Sμ/Sγ junction and into the Sγ switch region. Shared or baseline mutations were identified as base substitutions common to both time points but not present in the reference sequence. These mutations were relatively high in frequency in the JH region and very low in the enhancer region, with a slight increase in the Sμ region. These mutations also included deletions and insertions (1-20 bp). The shared mutations are presumably a result of somatic hypermutation and polymorphic variation in each patient, with some patients having higher mutation frequencies than others. New mutations were seen exclusively in second time point samples, at a relatively low frequency. In 3 of the 5 patients, the new mutation frequency is not significantly different from mutations due to Taq error (P < .02, P < .07, and P < .4 for MM2, MM5, and MM4, respectively, by chi-square test); in the 2 remaining patients, the new mutation frequency is significant (P < .001 for MM1 and MM3). The fact that the new mutations are identical in duplicates from distinct PCR reactions suggests that even the low frequency results are valid genetic changes that cannot be explained by Taq error. These mutations included base substitutions, deletions, and duplications. The most common mutation was single-base substitution, in which A>G mutations were most frequently observed, followed by T>C and T>G (Figure 4B). In 3 of 5 patients, mutations were seen in the enhancer region. Deletions were also observed in the later time point samples of 3 of 5 patients. Most notably, patient MM1 contains 3- and 64-bp deletions in the Iμ exon, the region where sterile transcripts originate,38 and patient MM3 has a 233-bp deletion of DNA that has been replaced by a tandem duplication of a 233-bp region immediately downstream. These mutations, especially the deletions, create substantial sequence changes in the V/D/J-S region of MM patients, and may provide markers to identify MM progenitors in future studies.
Detection of new mutations in the second time point sample of MM patients. (A) The positions of the germ-line elements in the V/D/J-S region are indicated at the top, with CS-PCR sequence from the diagnosis time point represented for patients MM1-5 below. Dashed lines represent regions that were not sequenced. New mutations in the second time point sample are shown as follows: closed circles are point mutations with base changes between first and second samples presented directly underneath; open circles, deletions, with the number of bases deleted shown above. A deletion followed by tandem duplication of downstream DNA is shown for MM3. (B) The frequency of each point mutation is summarized in the box.
Detection of new mutations in the second time point sample of MM patients. (A) The positions of the germ-line elements in the V/D/J-S region are indicated at the top, with CS-PCR sequence from the diagnosis time point represented for patients MM1-5 below. Dashed lines represent regions that were not sequenced. New mutations in the second time point sample are shown as follows: closed circles are point mutations with base changes between first and second samples presented directly underneath; open circles, deletions, with the number of bases deleted shown above. A deletion followed by tandem duplication of downstream DNA is shown for MM3. (B) The frequency of each point mutation is summarized in the box.
Discussion
In MM, several progenitor compartments have been proposed, including clonotypic B cells8 and PCs41,42 ; however, a definitive identification and characterization of an MM stem cell remains elusive. In previous work, we examined the role of clonotypic B cells in MM.7-11,18,22 Recent work by Kirshner et al has shown that CD20+ clonotypic B lymphocytes generate PCs and exhibit extensive self-renewal in a 3-D culture system.19 In this study, CS-PCR and DNA sequence analysis was used to determine whether cIgM cells play a direct role in replenishing postswitch MM-PC populations in MM.
We found that in all patients examined, a single unique clonotypic switch fragment predominated and persisted throughout the testing period, despite fluctuations in the malignant clone, regardless of whether it was depleted and subsequently regrew, remained stable, or expanded. A single switch junction persists at relapse, which represents a new growth phase of the MM clone, and in a patient progressing from MGUS to MM, which represents a change from a clinically nonmalignant condition to clinically overt MM. In the case of patients progressing from MGUS to MM, samples satisfying the criteria of this study, ones with a confirmed MGUS diagnosis, clonotypic V/D/J signature, and successful CS-PCR fragment amplification for both samples, have been difficult to obtain. A more detailed study of these patients may yet reveal a contribution of cIgM cells in progression from MGUS to MM. In terms of the cases studied here, the analysis argues against a direct role for cIgM cells in the generation of MM-PCs. Our CS-PCR testing method was straightforward and robust, being informative in the majority of cases, with a sensitivity of 1 in 104 for the single-stage reaction. Clonotypic switch junction sequence analysis in a subset of patients confirmed the CS-PCR results, revealing an unchanged switch junction with normal characteristics. It is possible that infrequent heterogeneous clonotypic switch fragments do exist in MM but remain undetectable by our analysis; however, there is no indication that heterogeneous CSR MM cells, if indeed present, show clonal outgrowth in later time point samples. Considering the unique nature of hybrid switch junctions generated during CSR, this sequence provides a second clonotypic signature, in addition to rearranged V/D/J, for MM cells. Although these results do not preclude a role for cIgM cells in stepwise transformation of postswitch PCs, they disfavor cIgM cells as an ongoing generative compartment for MM-PCs.
A second significant observation in this study was the occurrence of ongoing mutation in the V/D/J-S region of MM patients. For each patient, we developed a baseline mutation profile of the V/D/J-S region by comparing mutations in paired time point samples. In addition to the baseline profile, additional mutations were detected in later time point samples for the majority of patients tested. The baseline profile was characterized by extensive mutation of the JH region that decreased progressively toward the Sμ region, an expected result of somatic hypermutation at the IgH locus.43 This region lacked any large DNA lesions such as frequent deletions reported in B-cell chronic lymphocytic leukemia.44 The new mutations were relatively infrequent and interspersed throughout the V/D/J-S region in 5 of 6 patients. In 3 of these patients, the Sμ intronic enhancer region, an important element controlling immunoglobulin expression, was targeted. The mechanism responsible for these mutations remains unclear, although a proposed CSR-related DNA repair activity may account for the relatively high A to G mutation frequency observed in this study.45,46 The accumulation of mutations may alter the function of the enhancer and nearby regions (patient MM1), providing a selective advantage in mutated clones. Of equal importance, these mutations may provide a means of identifying postswitch populations that serve as MM progenitors. Unlike clonotypic V/D/J or switch junctions, V/D/J-S mutations change over time, providing means to monitor evolving MM cell populations and potentially pinpoint the origin of MM progenitors.
In our previous study on preswitch MM cells,22 we had proposed several explanations for the persistence of nonclinical isotypes in MM, namely (1) expression of both preswitch and postswitch isotypes by the same cell, through some form of aberrant CSR or trans-switching, (2) existence of discrete populations of preswitch and postswitch MM cells, and (3) existence of cIgM progenitors that undergo persistent, directed isotype switching and differentiation to MM-PCs. Of these, our current observations lend greater support for model 2, describing a discrete cIgM population that, although not directly responsible for generating MM-PCs, may be functionally tied to events within MM-PCs. For example, cIgM cells and MM-PCs may occupy similar BM niches, and respond in a similar fashion to growth signals and chemotherapy. The observation of strict intraclonal homogeneity,14,15,23 increased frequency in some MM patients,23 and engraftment capability in NOD/SCID mice,22 continues to suggest that cIgM cells are dysregulated in MM in a manner that remains to be determined. Currently, a novel 3-D model of MM BM, one that faithfully reconstructs the in vivo BM microenvironment and supports the growth of MM cells, is being analyzed to determine the fate of cIgM cells.19 Through this examination of MM cell interactions in a reconstructed BM environment, a better understanding of the role of cIgM cells in MM may emerge.
In summary, DNA fragment and sequence analysis of clonotypic switch PCR products suggests that postswitch MM originates from a single CSR event. Although cIgM cells persist throughout disease and are correlated with poor clinical outcomes, during disease progression they do not seem to contribute significantly to postswitched malignant PCs. In addition, we observed that in most patients mutations accumulate at a low frequency in the V/D/J-S region over time. These mutations may modulate enhancer-driven expression of downstream genes, providing a selective advantage for mutated clones. Furthermore, these mutations provide a new marker for studying MM clonal evolution and identifying MM-PC progenitors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the MM patients contributing to this study. We also thank Erin Strachan, Eva Baigorri, Tara Steffler, Juanita Wizniak, Darlene Paine, Alana Eshpeter, Jen Szydylowski, Sarah Motz, and Amanda Reichert for their expert technical assistance, and Jonathan Keats for valuable insights and discussion.
This work was funded by a grant from the Canadian Institutes for Health Research (CIHR, Ottawa, ON). T.R. is an Alberta Heritage Foundation for Medical Research (AHFMR, Edmonton, AB) Clinical Investigator. L.M.P. is Canada Research Chair in Biomedical Nanotechnology. This work was funded in part by the Chair's program.
Authorship
Contribution: B.J.T. designed and performed the research, analyzed data, and wrote the paper; J.K. assisted in experiment design and writing; J.A.P. performed research; M.J.M., T.R., and A.R.B. provided clinical input and contributed to writing the paper; and L.M.P. directed the research, assisted in data interpretation, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Linda M. Pilarski, Cross Cancer Institute, 11560 University Ave, Edmonton, AB, Canada T6G 1Z2; e-mail: lpilarsk@ualberta.ca.