Abstract
Rac GTPases have been implicated in the regulation of diverse functions in various blood cell lineages, but their role in T-cell development is not well understood. We have carried out conditional gene targeting to achieve hematopoietic stem cell (HSC)– or T-cell lineage–specific deletion of Rac1 or Rac1/Rac2 by crossbreeding the Mx-Cre or Lck-Cre transgenic mice with Rac1loxp/loxp or Rac1loxp/loxp;Rac2−/− mice. We found that (1) HSC deletion of both Rac1 and Rac2 inhibited production of common lymphoid progenitors (CLPs) in bone marrow and suppressed T-cell development in thymus and peripheral organs, whereas deletion of Rac1 moderately affected CLP production and T-cell development. (2) T cell–specific deletion of Rac1 did not affect T-cell development, whereas deletion of both Rac1 and Rac2 reduced immature CD4+CD8+ and mature CD4+ populations in thymus as well as CD4+ and CD8+ populations in spleen. (3) The developmental defects of Rac1/Rac2 knockout T cells were associated with proliferation, survival, adhesion, and migration defects. (4) Rac1/Rac2 deletion suppressed T-cell receptor–mediated proliferation, IL-2 production, and Akt activation in thymocytes. Thus, Rac1 and Rac2 have unique roles in CLP production and share a redundant but essential role in later stages of T-cell development by regulating survival and proliferation signals.
Introduction
Rac GTPases of the Rho family, Rac1, Rac2, and Rac3, are intracellular signal transducers.1-5 Rac1 is ubiquitously expressed, Rac2 is expressed only in hematopoietic cells, and Rac3 is expressed primarily in the brain.6-8 Previous studies have implicated Rac GTPases as key signaling components controlling actin cytoskeleton organization, and cell adhesion, migration, proliferation, and survival in mammalian cells,9-15 but the unique and redundant roles of individual Rac GTPases in T-cell development have yet to be vigorously investigated.
T lymphopoiesis initiates from common lymphoid progenitors (CLPs) in bone marrow, which migrate to thymus for further development.16 Maturation of T cells in thymus proceeds through a series of differentiation stages. The most immature populations in thymus comprise double-negative (DN) thymocytes (CD4−CD8−), which can be subdivided further to several stages depending on expression levels of CD44 and CD25. The differentiation of DN thymocytes to CD4+CD8+ double-positive (DP) cells is dependent on the expression and rearrangement of TCRβ and TCRα. DP cells undergo positive and negative selection, and differentiate to helper CD4+ or cytotoxic CD8+ single-positive (SP) T cells. The mature CD4+ or CD8+ SP T cells migrate to peripheral tissues (eg, spleen and peripheral blood) to mediate immune responses.17 Previous studies in transgenic mice expressing a constitutively active mutant of Rac1 found that Rac1 activity promotes the transition from DN3 to DN4 stages of T-cell differentiation and diverts thymocytes from positive to negative selection.18,19 However, transgenic mice expressing an activated mutant of Rac2 in the T-cell compartment showed massive apoptosis and depletion of CD4+CD8+ DP thymocytes and enhanced Th1 differentiation.20,21 It is not clear whether the difference of these studies is related to distinct Rac1 and Rac2 functions or reflects nonphysiologic responses resulting from overexpression of the mutants.
Recent studies using genetic approaches have shown that overexpression of constitutively active and/or dominant-negative mutants of Rac may have several limitations including disruption of normal signaling cross-talk between Rac and other GTPases,2-4 nonspecific sequestration of the upstream guanine nucleotide exchange factors, and promiscuous activation of multiple common effectors shared between Rac and other Rho GTPases. These effects can cause Rac-independent functional outcomes.22,23 It is thus highly desirable to use gene targeting approaches to assess Rac cell functions and the signaling requirements. In recent gene targeting studies, Rac2−/− mice showed impaired actin polymerization, Ca2+ generation, and Erk and p38 activation in CD4+ T cells. Furthermore, Rac2-deficient mice are defective in receptor clustering during T-cell stimulation and Th1 differentiation, whereas thymocyte development or positive/negative selection in these mice appears normal.24,25 Rac1 conditional knockout mice have also displayed abnormalities in a variety of blood cells including neutrophils, hematopoietic stem/progenitor cells, macrophages, B cells, and dendritic cells.26-31 To date, the role of Rac1, particularly the distinct versus redundant role compared with that of Rac2, in T-cell development, remains to be elucidated.
Here we have used gene-targeted Rac1loxp/loxp mice to generate Rac1-deficient and Rac1/Rac2-double deficient T lymphocytes in a hematopoietic stem cell–inducible knockout mouse model and a T-cell lineage–specific knockout mouse model. We show that deletion of Rac1 alone has limited effect on the developmental steps of T cells except the CLP stage. However, deletion of both Rac1 and Rac2 significantly affects the immature CD4−CD8− and CD4+CD8+ populations as well as the mature CD4+ and CD8+ SP populations in the thymus and/or spleen. The developmental defects of Rac1/Rac2 knockout T cells are associated with a proliferation defect and increased apoptosis. The results demonstrate that Rac1 and Rac2 play redundant but critical roles during multiple stages of T-cell development by regulating survival and proliferation signals.
Methods
Mouse gene targeting
Conditional gene-targeted Rac1loxp/loxp mice in C57Bl/6 background were generated as described previously.26-31 The flox allele contains loxP sites flanking exon 1 of Rac1 gene (Figure 1A). Rac1loxp/loxp mice were bred to Rac2−/− mice to generate Rac1loxp/loxp/Rac2−/− mice. To delete Rac1 in vivo in hematopoietic stem cells, Mx-Cre+/Rac1loxp/loxp or Mx-Cre+/Rac1flox/flox/Rac2−/− mice were generated by breeding Rac1loxp/loxp or Rac1loxp/loxp/Rac2−/− mice to Mx-Cre+ transgenic mice carrying a bacteriophage Cre recombinase driven by an interferon-γ–inducible Mx1 promoter (gift from Dr Stuart H. Orkin, Dana-Farber Cancer Institute, Boston, MA) (Figure 1B). The expression of Mx-Cre was induced by 4 to 5 intraperitoneal injections of 300 μg poly I:C (Amersham Pharmacia Biotech, Piscataway, NJ) into Mx-Cre+/Rac1loxp/loxp or Mx-Cre+/Rac1loxp/loxp/Rac2−/− mice at 2-day intervals.27,28 To delete Rac1 in vivo in the T-cell lineage, Lck-Cre+/Rac1loxp/loxp or Lck-Cre+/Rac1loxp/loxp/Rac2−/− mice were generated by crossing Rac1loxp/loxp or Rac1loxp/loxp/Rac2−/− mice to transgenic mice expressing Cre recombinase under the direction of Lck proximal promoter (obtained from The Jackson Laboratory, Bar Harbor, ME; Figure 4A). The genotyping protocols were as described in Gu et al28 or as recommended by The Jackson Laboratory.
Immunoblotting
For immunoblotting of Rac1, whole-cell lysates were prepared by extraction of the bone marrow cells, thymocytes, and/or splenocytes in a lysis buffer containing 20 mM Tris-HCl (pH 7.6), 100 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 0.2% sodium deoxycholate, 2 mM phenylmethylsulfonyl fluoride, 10 μg leupeptin/mL, 10 μg aprotinin/mL, and 0.5 mM dithiothreitol for 30 minutes. Protein contents in the whole-cell lysates were normalized by the Bradford method. The lysates were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis.32 The expression of Rac1 was probed using an anti-Rac1 antibody (Upstate Biotechnology, Lake Placid, NY).
For immunoblotting of phospho- or total Akt, Erk, p38, and ZAP70, isolated thymocytes were incubated with or without anti-TCR mAb (H57-597) 10 μg/mL on ice for 30 minutes. The cells were stimulated with anti-IgG at 30 μg/mL for 3 minutes. Whole-cell lysates were prepared and protein contents in the whole-cell lysates were normalized. The lysates were blotted for phospho- or total Akt, Erk, p38, or ZAP70 using respective antibodies (Cell Signaling, Beverly, MA).
Flow cytometry and complete blood count
Antibodies for flow cytometry, anti-CD3, -TCRβ, -CD4, -CD8, -B220, -Gr1, -CD11b, -TER119, -IL7Rα, –c-Kit, -Sca1, -CD69, -CD44, and -CD25, were purchased from BD Pharmingen (San Diego, CA). Fluorescence-activated cell sorting (FACS) analysis was performed on a FACSCanto system using FACSDiVa software (BD Biosciences, San Jose, CA). Automated complete blood counts were performed using a Hemavet 850FS (Drew Scientific, Dallas, TX).
Quantification of cell numbers
Cell number of T-cell subpopulations in thymus or spleen was determined by multiplying the percentage of the respective T-cell subsets as measured by FACS by the total number of thymocytes or splenocytes. T-cell or B-cell number in peripheral blood was determined by multiplying the percentages of T cells or B cells in peripheral blood as determined from FACS analysis of CD3+ or TCRβ+ T cells or B220+ B cells by the total number of white blood cells from complete blood count. For calculation of thwere stained for lineage markers with biotinylated antibodies against B220, CD3, CD4, CD8, Gr1, CD11b, and TER119. Subsequently, cells were stained with Streptavidin-Percp and anti–IL7Rα-APC-Cy7, –c-kit-APC, and –Sca1-PE antibodies. The percentage of CLPs of Lin−IL-7Rα+Sca1medc-kitmed-high cells was analyzed by FACS.33 The number of CLPs was determined by multiplying the percentage of CLP by the total number of bone marrow cells.
Cell apoptosis analysis
Isolated thymocytes or splenocytes were incubated with anti-CD4, anti-CD8, and/or anti-TCRβ antibodies together with annexin V (BD Pharmingen) followed by a FACS analysis.33 T-cell subpopulations in thymocytes or in splenocytes were gated to determine the percentage of annexin V+ cells.
In vivo BrdU incorporation
Mice were injected intraperitoneally with 100 μg/g body weight of BrdU (Sigma-Aldrich, St Louis, MO). Twelve hours after the BrdU injection, thymocytes or splenocytes were isolated, incubated with antibodies against CD4, CD8, and/or TCRβ, fixed, permeabilized, and incubated with anti-BrdU antibody (Sigma-Aldrich).34 All experimentation procedures using animals were approved by the Cincinnati Children's Hospital Medical Center Institutional Animal Control and Usage Committee (protocol number 8D06052) and abide by National Institutes of Health (NIH, Bethesda, MD) guidelines.
Cell adhesion assay
Splenic T cells were purified by a pan T-cell purification kit (Miltenyi Biotec, Auburn, CA). Thymocytes or splenic T cells (105) were plated onto 10 μg/mL fibronectin-coated 96-well plates and incubated for 2 hours. The unattached cells were washed away with Dulbecco modified phosphate-buffered saline. The attached cells were trypsinized and quantified.
Cell migration assay
Cell migration was measured using a transwell chamber. Dulbecco modified Eagle medium (DMEM) containing 500 ng/mL SDF-1 (PEPROTECH, Rocky Hill, NJ) or MIP-3β (R&D System, Minneapolis, MN) was added to the exterior of the transwell chamber. Total thymocytes, CD4+ or CD8+ SP thymocytes, or splenic T cells (105) were suspended in the DMEM and added to the interior of the transwell chamber and incubated for 4 hours. Cells migrated through polyester membrane were visualized by Giemsa staining and counted.
Cell proliferation assay
Equal amounts of isolated thymocytes or splenic T cells were plated on 96-well plates with or without precoating of anti-TCR mAb (H57-597) (BD Pharmingen). IL-2 production was determined using an enzyme-linked immunosorbent assay (ELISA) kit (eBioscience, San Diego, CA). Cell counts were performed at day 3 in culture.
Results
Gene targeting of Rac1 or Rac1/Rac2 in hematopoietic stem cells
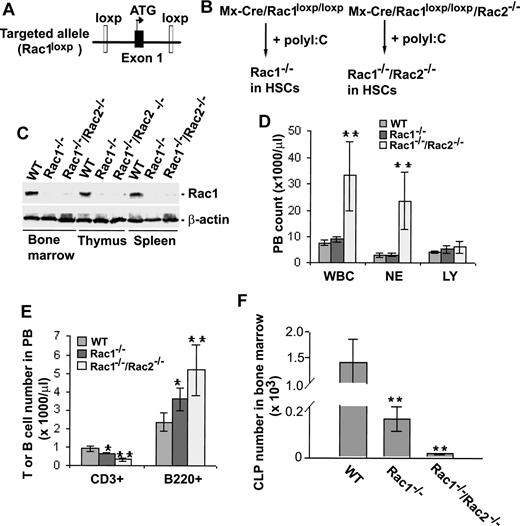
To investigate the role of Rac1 and Rac2 in T-cell development starting from hematopoietic stem cells, we used previously generated Mx-Cre/Rac1loxp/loxp or Mx-Cre/Rac1loxp/loxp/Rac2−/− mice that allow effective deletion of Rac1 gene after poly I:C–induced inflammatory reaction in the mice27,28 (Figure 1A,B). Bone marrow cells, thymocytes, and splenocytes were collected from these mice and the matching WT control mice 15 to 20 days after 4 to 5 consecutive poly I:C injections and were subjected to anti-Rac1 Western blotting. As shown in Figure 1C, removal of Rac1 protein in bone marrow, thymocytes, and splenocytes of Mx-Cre/Rac1loxp/loxp or Mx-Cre/Rac1loxp/loxp/Rac2−/− was achieved at approximately 90% to 95% efficiency under these conditions. Rac2 gene and Rac2 protein were absent in the treated Mx-Cre/Rac1loxp/loxp/Rac2−/− mice (data not shown). These animals were designated Rac1−/− or Rac1−/−/Rac2−/− in hematopoietic stem cells (HSCs) for the following characterizations.
Gene targeting of Rac1 or Rac1/Rac2 in HSCs and the effect on peripheral blood and bone marrow cells. (A) Rac1 gene–targeted allele. The conditional Rac1 allele was generated by sandwiching exon 1 of Rac1 gene with 2 loxP sites. (B) Generation of Rac1 or Rac1/Rac2 knockout HSCs in mice. To produce Rac1- or Rac1/Rac2-deficient hematopoietic cells in mice, Rac1loxp/loxp or Rac1loxp/loxp/Rac2−/− mice were crossbred with Mx-Cre transgenic mice. Four to 5 doses of poly I:C injection of the mice resulted in efficient deletion of Rac1 gene in HSCs. (C) Expression of Rac1 in bone marrow, thymus, and spleen of Rac1 or Rac1/Rac2 Mx-Cre–targeted mice. Bone marrow cells, thymocytes, and splenocytes from WT-, Rac1-, or Rac1/Rac2-targeted mice were probed for Rac1 protein by anti-Rac1 blotting. Levels of β-actin were used as loading controls. (D) Deletion of Rac1/Rac2 but not Rac1 alone led to increased white blood cells (WBCs) and neutrophils (NEs) without apparent effect on lymphocyte numbers (LY) in peripheral blood. PB of various genotypes was analyzed by blood counting on a hematology analyzer. (E) PB of various genotypes was stained by biotinylated anti-CD3 or -B220 antibody followed by Streptavidin-Percp and analyzed by flow cytometry. (F) Deletion of Rac1 or Rac1/Rac2 reduced CLP numbers in bone marrow. Bone marrow cells were counted and stained for lineage markers with biotinylated antibodies against B220, CD3, CD4, CD8, Gr1, CD11b, and TER119. Subsequently, cells were stained with Streptavidin-Percp, anti–IL7R-APC-Cy7, anti–c-kit-APC, and anti–Sca1-PE. CLP was defined by the Lin−IL-7Rα+Sca1medc-kitmed-high phenotype. The number of CLP was calculated based on the total number of bone marrow cells and the percentage of CLP. n = 5 in each panel. *P < .05; **P < .01. Error bars represent SD.
Gene targeting of Rac1 or Rac1/Rac2 in HSCs and the effect on peripheral blood and bone marrow cells. (A) Rac1 gene–targeted allele. The conditional Rac1 allele was generated by sandwiching exon 1 of Rac1 gene with 2 loxP sites. (B) Generation of Rac1 or Rac1/Rac2 knockout HSCs in mice. To produce Rac1- or Rac1/Rac2-deficient hematopoietic cells in mice, Rac1loxp/loxp or Rac1loxp/loxp/Rac2−/− mice were crossbred with Mx-Cre transgenic mice. Four to 5 doses of poly I:C injection of the mice resulted in efficient deletion of Rac1 gene in HSCs. (C) Expression of Rac1 in bone marrow, thymus, and spleen of Rac1 or Rac1/Rac2 Mx-Cre–targeted mice. Bone marrow cells, thymocytes, and splenocytes from WT-, Rac1-, or Rac1/Rac2-targeted mice were probed for Rac1 protein by anti-Rac1 blotting. Levels of β-actin were used as loading controls. (D) Deletion of Rac1/Rac2 but not Rac1 alone led to increased white blood cells (WBCs) and neutrophils (NEs) without apparent effect on lymphocyte numbers (LY) in peripheral blood. PB of various genotypes was analyzed by blood counting on a hematology analyzer. (E) PB of various genotypes was stained by biotinylated anti-CD3 or -B220 antibody followed by Streptavidin-Percp and analyzed by flow cytometry. (F) Deletion of Rac1 or Rac1/Rac2 reduced CLP numbers in bone marrow. Bone marrow cells were counted and stained for lineage markers with biotinylated antibodies against B220, CD3, CD4, CD8, Gr1, CD11b, and TER119. Subsequently, cells were stained with Streptavidin-Percp, anti–IL7R-APC-Cy7, anti–c-kit-APC, and anti–Sca1-PE. CLP was defined by the Lin−IL-7Rα+Sca1medc-kitmed-high phenotype. The number of CLP was calculated based on the total number of bone marrow cells and the percentage of CLP. n = 5 in each panel. *P < .05; **P < .01. Error bars represent SD.
Hematopoietic deletion of Rac1 results in decreased bone marrow lymphopoiesis and T-cell circulation
To begin to explore the roles of Rac1 and Rac2 in T-cell development, we analyzed lymphocyte and T-cell populations in peripheral blood from poly I:C–treated Mx-Cre/Rac1loxp/loxp or Mx-Cre/Rac1loxp/loxp/Rac2−/− mice. Complete blood count showed that loss of Rac1 or loss of Rac1/Rac2 had little effect on the number of total lymphocytes (Figure 1D). However, loss of Rac1/Rac2, not Rac1 alone, dramatically increased white blood cell (WBC) and neutrophil (NE) counts (Figure 1D), similar to what has been reported previously.28,29,35 Further FACS analyses of T- and B-cell populations in peripheral blood by staining with anti-CD3 and anti-B220 antibodies demonstrated that deletion of both Rac1 and Rac2 caused a significant decrease in T-cell number accompanied by an increase in B-cell number (Figure 1E). Deletion of Rac1 alone also produced a similar effect on T-cell and B-cell numbers, albeit to a lesser extent (Figure 1E). Since deletion of Rac2 results in an increase in T-cell number in peripheral blood25 (and data not shown), it appears that a balance between the decrease of T-cell number by Rac1 deficiency and an increase of T-cell number by Rac2 deficiency was shifted toward a decrease of T-cell population in the Rac1/Rac2 double-deficient mice. Moreover, Rac2 deletion was reported to result in a modest increase in B-cell number in peripheral blood,36 which may contribute to the observed increase of B cells in Rac1/Rac2-deficient mice additively to that of Rac1 deletion. Overall, the seemingly normal level of lymphocyte number in PB of Rac1−/− and Rac1−/−/Rac2−/− mice appears to be a net effect of increased B cells and reduced T cells caused by Rac1 or Rac1/Rac2 deficiency.
Since removal of Rac1 or Rac1/Rac2 from HSCs reduced T-cell numbers in peripheral blood, we next investigated whether the progenitors of T-cell lineage in the bone marrow were affected by the respective Rac gene targeting. Analysis of the BM content revealed that Rac1 deletion caused an approximately 8-fold decrease in the common lymphoid progenitor (CLP, Lin−/IL-7Ra+) number, whereas Rac1/Rac2 deletion caused approximately 20-fold reduction of the CLP number (Figure 1F), and the total bone marrow cellularities and HSCs of Rac1−/− and Rac1−/−/Rac2−/− mice decreased by only approximately 2- and 3-fold, respectively28,29 (and data not shown). These results indicate that Rac1 and Rac2 play an essential and additive role in HSC differentiation to CLP in the bone marrow.
Hematopoietic induction of Rac1 deficiency synergizes with Rac2 deficiency to suppress post-DN T-cell differentiation
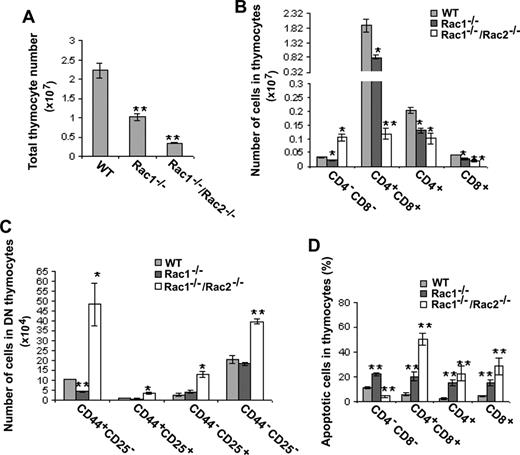
Since thymus is the main organ where pre-T cells develop into mature T cells, we further analyzed T-cell development in the thymus of mutant mice. Consistent with the marked reduction of CLPs, the Rac1- or Rac1/Rac2-targeted mice displayed significantly reduced thymus size (data not shown). The total number of thymocytes of Rac1−/− and Rac1−/−/Rac2−/− mice was reduced by approximately 50% and approximately 85%, respectively (Figure 2A). Quantification of T-cell subpopulations in the thymocytes by FACS showed that CD4−CD8− double-negative (DN) cell number was reduced by approximately 35% in Rac1−/− thymus but increased by approximately 3-fold in Rac1−/−/Rac2−/− thymocytes (Figure 2B). Further analysis of DN subpopulations by staining for CD44 and CD25 found a decrease of DN1 cells (CD44+CD25−) in Rac1−/− mice and an increase of all 4 DN subpopulations in Rac1−/−/Rac2−/− mice (Figure 2C), suggesting that Rac1 and Rac2 play distinct roles in DN differentiation. Rac1 deficiency led to more than 50% reduction in CD4+CD8+ double-positive (DP) T-cell number, whereas Rac1/Rac2 deficiency resulted in an almost complete blockage of DP T-cell development. The later stage of single-positive (SP) selections for CD4+CD8− and CD4−CD8+ T-cell subpopulations was similarly inhibited by Rac1 or Rac1/Rac2 deficiency (Figure 2B). Together with previous observations that Rac2 deficiency does not detectably affect thymocyte T-cell subpopulation distribution25 (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), these results indicate that Rac1 and Rac2 play a redundant but essential role in the T-cell development in the thymus.
Deletion of Rac1 or Rac1/Rac2 in HSCs affects T-cell development and survival in thymus. (A) Thymic cellularity of Mx-Cre–targeted mice was determined in the indicated genotypes. Single-cell suspensions were prepared from freshly isolated thymus for quantification. (B) The numbers of T-cell subpopulations in thymocytes were determined by anti-CD4, -CD8, -CD44, and -CD25 staining and FACS analysis. (C) The numbers of DN thymocyte subpopulations were determined using additional anti-CD44 and anti-CD25 antibodies and subsequent FACS analysis. (D) The apoptotic cells in thymic T-cell subpopulations were determined by anti-CD4 and -CD8 and annexin V staining followed by flow cytometry. The results are representative of 2 experiments. n = 5 for each genotype. *P < .05; **P < .01. Error bars represent SD.
Deletion of Rac1 or Rac1/Rac2 in HSCs affects T-cell development and survival in thymus. (A) Thymic cellularity of Mx-Cre–targeted mice was determined in the indicated genotypes. Single-cell suspensions were prepared from freshly isolated thymus for quantification. (B) The numbers of T-cell subpopulations in thymocytes were determined by anti-CD4, -CD8, -CD44, and -CD25 staining and FACS analysis. (C) The numbers of DN thymocyte subpopulations were determined using additional anti-CD44 and anti-CD25 antibodies and subsequent FACS analysis. (D) The apoptotic cells in thymic T-cell subpopulations were determined by anti-CD4 and -CD8 and annexin V staining followed by flow cytometry. The results are representative of 2 experiments. n = 5 for each genotype. *P < .05; **P < .01. Error bars represent SD.
Apoptosis has been shown to be important for T-cell development.16 One possible mechanism of the abnormal T-cell development in the thymus caused by deletion of Rac1 or Rac1/Rac2 from hematopoietic stem cells is deregulated apoptosis at the various developmental stages. Examination of DN, DP, and SP T-cell subsets in mutant thymocytes revealed that DN thymocytes from Rac1-deficient mice as well as DP and SP thymocytes were more susceptible to apoptosis (Figure 2D). Whereas deletion of both Rac1 and Rac2 resulted in significantly more apoptotic DP and SP cells than that by deletion of Rac1 alone, Rac1/Rac2 deletion led to decreased, rather than increased, DN apoptotic thymocytes compared with WT cells (Figure 2D). The apoptotic T-cell subpopulation distributions of the Rac1 and Rac1/Rac2 mutants correlate with the increase or decrease of the cellularities of T-cell subpopulations in thymocytes, and are consistent with the possibility that Rac1 and Rac2 are additively involved in regulating DP and SP T-cell survival and production in thymus.
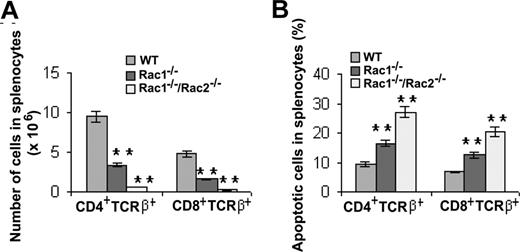
Next, the content of postthymic differentiated T cells was analyzed in secondary lymphoid organs. Spleens of Rac1 and Rac1/Rac2 mutant mice contained approximately one-third and approximately one-tenth, respectively, of both CD4+TCRβ+ and CD8+TCRβ+ T cells compared with WT mice (Figure 3A). The decrease of the mature T-cell numbers in the mutant mice inversely correlated with their susceptibility to apoptosis, as Rac1- and Rac1/Rac2-deficient T cells in splenocytes displayed significantly increased apoptosis that appears to additively depend on the loss of Rac1 and Rac2 (Figure 3B). These results suggest that Rac1 and Rac2 provide additive signals essential for mature T-cell survival and cell number regulation in spleen. In general, the role of Rac1 and Rac2 in splenic T-cell production appears similar to that in thymocyte DP and SP T-cell development and survival (Figure 2).
Deletion of Rac1 or Rac1/Rac2 in HSCs results in decreased T-lymphocyte cellularity in spleen that is associated with increased apoptosis. (A) Number of T cells in splenocytes was determined by staining the splenocytes with anti-CD4, -CD8, and -TCRβ antibodies followed by flow cytometry. (B) The apoptotic T-cell subpopulations in splenocytes were measured by the antibody and annexin V costaining followed by flow cytometry. The results are representative of 2 experiments. n = 5 for each genotype. **P < .01. Error bars represent SD.
Deletion of Rac1 or Rac1/Rac2 in HSCs results in decreased T-lymphocyte cellularity in spleen that is associated with increased apoptosis. (A) Number of T cells in splenocytes was determined by staining the splenocytes with anti-CD4, -CD8, and -TCRβ antibodies followed by flow cytometry. (B) The apoptotic T-cell subpopulations in splenocytes were measured by the antibody and annexin V costaining followed by flow cytometry. The results are representative of 2 experiments. n = 5 for each genotype. **P < .01. Error bars represent SD.
Gene targeting of Rac1 or Rac1/Rac2 in the T-cell lineage
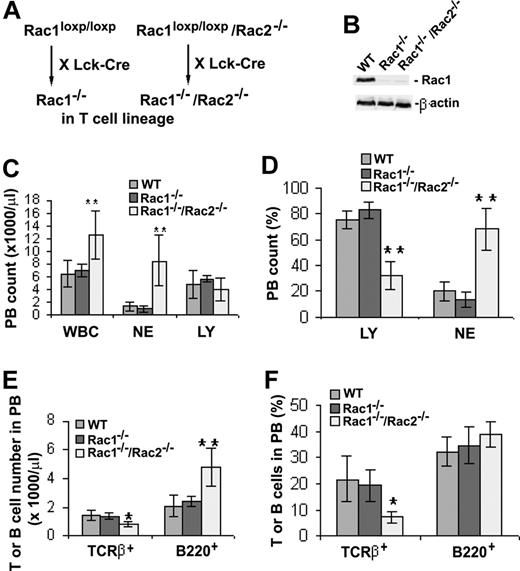
The studies of HSC-targeted deletion of Rac1 or Rac1/Rac2 suggest that Rac1 and Rac2 play a partially redundant but essential role in CLP production and in DP and SP T-cell development. However, because Mx-Cre–mediated Rac1 deletion in HSCs affects multilineages of blood cell development and causes a profound inhibitory effect on CLP generation that may mask the role of Rac1 and/or Rac2 in later T-cell developmental stages, we next generated T cell–targeted mice by crossbreeding Rac1loxp/loxp or Rac1loxp/loxp/Rac2−/− mice with Lck-Cre transgenic mice to achieve Rac1 and Rac1/Rac2 deficiency in the T-cell lineage (Figure 4A). As shown in Figure 4B, Rac1 expression in the Lck-Cre+/Rac1loxp/loxp or Lck-Cre/Rac1loxp/loxp/Rac2−/− mouse thymocytes was reduced by more than 90%. These animals were designated as Rac1−/− or Rac1−/−/Rac2−/− in the T-cell lineage in the following studies.
Gene targeting of Rac1 or Rac1/Rac2 in the T-cell lineage and the effect on peripheral blood cells. (A) Generation of T cell–specific Rac1- or Rac1/Rac2-deficient mice. To produce Rac1- or Rac1/Rac2-deficient T lymphocytes, Rac1loxp/loxp or Rac1loxp/loxp/Rac2−/− mice were crossbred with Lck-Cre transgenic mice. (B) Expression of Rac1 in Lck-Cre–targeted thymocytes. WT, Rac1−/−, or Rac1−/−Rac2−/− thymocytes were probed for Rac1 protein expression by anti-Rac1 Western blotting. Levels of β-actin in each sample were probed as loading controls. (C) Deletion of Rac1/Rac2, but not Rac1 alone, led to increased WBC and NE counts but normal LY counts in peripheral blood. (D) Deletion of Rac1/Rac2 but not Rac1 by Lck-Cre targeting caused decreased LY percentage and increased NE percentage in peripheral blood cells. (E,F) The effects of Rac1 or Rac1/Rac2 deletion on T- and B-cell productions in peripheral blood. PB cells were stained for TCRβ or B220 and analyzed by flow cytometry. The T- and B-cell numbers and percentages in PB were presented in panels E and F, respectively. n = 7 for each genotype. *P < .05; **P < .01. Error bars represent SD.
Gene targeting of Rac1 or Rac1/Rac2 in the T-cell lineage and the effect on peripheral blood cells. (A) Generation of T cell–specific Rac1- or Rac1/Rac2-deficient mice. To produce Rac1- or Rac1/Rac2-deficient T lymphocytes, Rac1loxp/loxp or Rac1loxp/loxp/Rac2−/− mice were crossbred with Lck-Cre transgenic mice. (B) Expression of Rac1 in Lck-Cre–targeted thymocytes. WT, Rac1−/−, or Rac1−/−Rac2−/− thymocytes were probed for Rac1 protein expression by anti-Rac1 Western blotting. Levels of β-actin in each sample were probed as loading controls. (C) Deletion of Rac1/Rac2, but not Rac1 alone, led to increased WBC and NE counts but normal LY counts in peripheral blood. (D) Deletion of Rac1/Rac2 but not Rac1 by Lck-Cre targeting caused decreased LY percentage and increased NE percentage in peripheral blood cells. (E,F) The effects of Rac1 or Rac1/Rac2 deletion on T- and B-cell productions in peripheral blood. PB cells were stained for TCRβ or B220 and analyzed by flow cytometry. The T- and B-cell numbers and percentages in PB were presented in panels E and F, respectively. n = 7 for each genotype. *P < .05; **P < .01. Error bars represent SD.
Similar to HSC-targeted deletion of Rac1/Rac2, deletion of both Rac1 and Rac2 from T-cell lineage caused a marked increase in WBC and NE counts without significantly affecting lymphocyte (LY) counts (Figure 4C). The percentage of LY in PB of Rac1/Rac2-deficient mice decreased, whereas that of NE increased, compared with WT mice (Figure 4D). Rac1 deletion alone did not have any significant impact on WBC, NE, or LY counts in PB. The unaltered LY counts in Lck-Cre+/Rac1loxp/loxp/Rac2−/− mice may be due to a counterbalance between a decrease in T-cell number and an increase in B-cell number (Figure 4E). However, only the mature TCRβ+ T-cell percentage in PB, not the B220+ B-cell percentage, was affected by Rac1/Rac2 deficiency (Figure 4F). Since Lck-Cre–targeted Rac1 deletion occurs only in T cells, the increase of NE and B-cell numbers in Lck-Cre–mediated, Rac1/Rac2-deficient mice is likely resulted from constitutive Rac2 deletion as reported previously for the Rac2−/− mice.35,36 Furthermore, different from the observation that Rac1 deletion alone from HSCs led to decreased T-cell number and increased B-cell number in peripheral blood, deletion of Rac1 alone from T-cell lineage did not affect T-cell or B-cell numbers in peripheral blood (Figure 4E,F). These results indicate that Rac1 and Rac2 play overlapping roles in the regulation of mature T-cell development.
Gene targeting in T-cell lineage unveils essential roles of Rac1/Rac2 in T-cell development
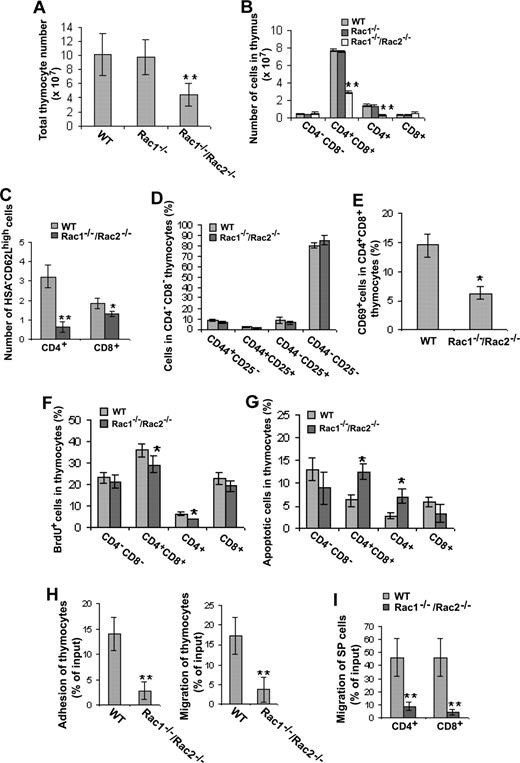
From the total thymocyte cellularity and CD4/CD8 DN, DP, or SP quantifications, it is clear that deletion of Rac1 alone from T-cell lineage had little effect on T-cell development in thymus, whereas deletion of both Rac1 and Rac2 caused a significant decrease in thymocyte cellularity (Figure 5A) and in the DP and CD4+ SP cell populations (Figure 5B). Although the number of CD8+ SP cells was not affected by the Rac1/Rac2 deficiency (Figure 5B), the frequency of mature CD8+ SP cells (CD8+ HSA-CD62L high) as well as mature CD4+ SP cells (CD4+ HSA-CD62L high) was significantly decreased (Figure 5C).
Deletion of Rac1/Rac2, but not Rac1 alone, in T cells affects the development and proliferation/survival/adhesion/migration of thymocyte T cells. Lck-Cre–targeted mice were injected intraperitoneally with BrdU (100 μg/g body weight) 12 hours prior to harvesting thymus. Isolated thymocytes were quantified for the absolute number (A) and stained with anti-CD4, -CD8, -CD44, -CD25, -CD69, -HSA, -CD62L, and/or -BrdU antibodies or annexin V. The stained cells were subjected to flow cytometry analysis of the CD4/CD8 subpopulations (B), the expression level of HSA/CD62L in CD4+ or CD8+ SP thymocytes (C), the CD44/CD25 expression in CD4−CD8− DN subpopulations (D), the CD69 level in CD4+CD8+ DP thymocytes (E), the proliferating cells (F), and the apoptotic cells (G) in various T-cell subpopulations. The adhesion to fibronectin and migration to SDF-1α of total thymocytes (H) or migration of CD4+ or CD8+ SP thymocyte to MIP-3β (I) was also determined. WT, n = 10; Rac1−/−, n = 6; and Rac1−/−Rac2−/−, n = 6. *P < .05; **P < .01. Error bars represent SD.
Deletion of Rac1/Rac2, but not Rac1 alone, in T cells affects the development and proliferation/survival/adhesion/migration of thymocyte T cells. Lck-Cre–targeted mice were injected intraperitoneally with BrdU (100 μg/g body weight) 12 hours prior to harvesting thymus. Isolated thymocytes were quantified for the absolute number (A) and stained with anti-CD4, -CD8, -CD44, -CD25, -CD69, -HSA, -CD62L, and/or -BrdU antibodies or annexin V. The stained cells were subjected to flow cytometry analysis of the CD4/CD8 subpopulations (B), the expression level of HSA/CD62L in CD4+ or CD8+ SP thymocytes (C), the CD44/CD25 expression in CD4−CD8− DN subpopulations (D), the CD69 level in CD4+CD8+ DP thymocytes (E), the proliferating cells (F), and the apoptotic cells (G) in various T-cell subpopulations. The adhesion to fibronectin and migration to SDF-1α of total thymocytes (H) or migration of CD4+ or CD8+ SP thymocyte to MIP-3β (I) was also determined. WT, n = 10; Rac1−/−, n = 6; and Rac1−/−Rac2−/−, n = 6. *P < .05; **P < .01. Error bars represent SD.
A further examination of CD44 and CD25 expression in CD4−CD8− DN cells found no detectable alterations in all 4 DN subpopulations in Rac1−/−/Rac2−/− mice compared with that in WT mice (Figure 5D), suggesting that Rac1 and Rac2 deficiency has no effect on β-selection in this mouse model. However, it remains a possibility that the lack of effects of Rac1/Rac2 deletion on the DN population is due to delayed timing of Rac1 deletion by the Lck-Cre system in use. To investigate whether there is a defect in positive selection in Rac1−/−/Rac2−/− mice, we analyzed CD69 expression in CD4+CD8+ DP thymocytes, the level of which is transiently increased during positive selection.37 As shown in Figure 5E, disruption of Rac1 and Rac2 caused a significant reduction of CD69 expression in CD4+CD8+ DP cells, indicating an impaired positive selection in the mutant mice.
By measuring in vivo BrdU incorporation, we found that the subpopulations of DP and CD4+ SP from the Rac1−/−/Rac2−/− thymocytes displayed moderately reduced proliferative potential (Figure 5F). Moreover, DP and CD4+ SP cells showed increased apoptotic cell proportions (Figure 5G). It appears that the decreased numbers of DP and CD4+ SP cells in Rac1−/−/Rac2−/− mice reflect a combinatory effect of decreased proliferation and survival capabilities.
Cell adhesion and migration in thymus are critical for thymopoiesis. We found that Rac1/Rac2 depletion caused a defect in adhesion of thymocytes to fibronectin and migration toward SDF-1α (Figure 5H). Moreover, Rac1/Rac2-deficient CD4+ and CD8+ SP cells were defective in migration to MIP-3β (Figure 5I), indicating that thymic output of CD4+ and CD8+ cells might be impaired in the mice.
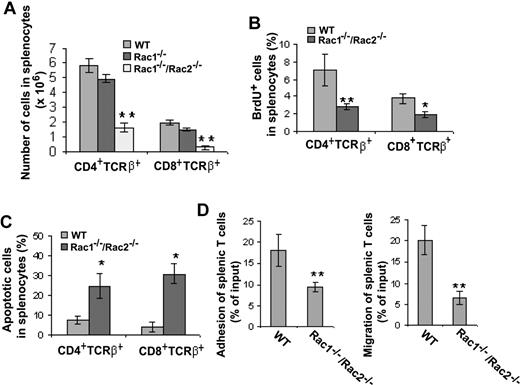
In the spleen, deletion of either Rac1 or Rac1/Rac2 from T-cell lineage had no effect on total splenocyte cell number (data not shown), but deficiency of both Rac1 and Rac2 induced a significant reduction of splenic CD4+ and CD8+ T cells (Figure 6A). BrdU labeling of Rac1/Rac2-deficient splenic T cells revealed a clear decrease in proliferation of both CD4+ and CD8+ cells (Figure 6B), whereas annexin V staining of the same T-cell populations showed significantly increased apoptosis (Figure 6C). Moreover, deficiency of Rac1/Rac2 in splenic T cells led to an approximately 50% reduction of adhesion to fibronectin and approximately 70% reduction of migration toward SDF-1α (Figure 6D). These data suggest that combined effects of Rac1/Rac2 deficiency on T-cell proliferation, survival, adhesion, and migration may contribute to the reduced mature T-cell numbers in the Rac1/Rac2 knockout mice.
Deletion of Rac1/Rac2, but not Rac1 alone, in T cells results in decreased T-lymphocyte cellularity in spleen that is associated with decreased T-cell proliferation, adhesion, and migration and increased apoptosis. Lck-Cre–targeted mice were injected intraperitoneally with BrdU (100 μg/g body weight) 12 hours prior to spleen harvest. The isolated splenocytes were counted and stained with anti-CD4, -CD8, -TCRβ, and -BrdU antibodies or annexin V followed by flow cytometry analysis of the CD4/CD8 subpopulations (A), the proliferating cells (B), and the apoptotic cells (C) in various T-cell subpopulations. The purified splenic T cells were also assayed for adhesion to fibronectin and for migration toward SDF-1α (D). WT, n = 10; Rac1−/−, n = 6; and Rac1−/−Rac2−/−, n = 6. *P < .05; **P < .01. Error bars represent SD.
Deletion of Rac1/Rac2, but not Rac1 alone, in T cells results in decreased T-lymphocyte cellularity in spleen that is associated with decreased T-cell proliferation, adhesion, and migration and increased apoptosis. Lck-Cre–targeted mice were injected intraperitoneally with BrdU (100 μg/g body weight) 12 hours prior to spleen harvest. The isolated splenocytes were counted and stained with anti-CD4, -CD8, -TCRβ, and -BrdU antibodies or annexin V followed by flow cytometry analysis of the CD4/CD8 subpopulations (A), the proliferating cells (B), and the apoptotic cells (C) in various T-cell subpopulations. The purified splenic T cells were also assayed for adhesion to fibronectin and for migration toward SDF-1α (D). WT, n = 10; Rac1−/−, n = 6; and Rac1−/−Rac2−/−, n = 6. *P < .05; **P < .01. Error bars represent SD.
T-cell growth induced by T-cell receptor cross-linking depends on Rac1/Rac2 signaling
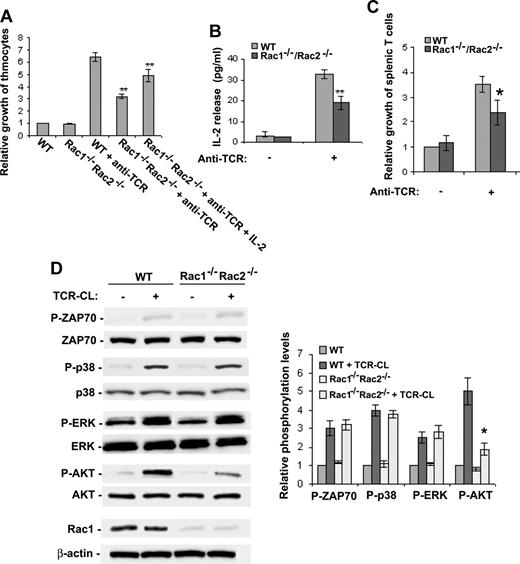
The phenotypic examinations provide evidence that Rac1 and Rac2 redundantly regulate T-cell maturation. To further understand the potential mechanisms underlying the Rac1/Rac2-regulated T-cell development, we examined cell growth rate of thymocytes upon TCR activation. As shown in Figure 7A, TCR activation by anti-TCR antibody cross-linking increased thymocyte growth by more than 6-fold, whereas deletion of Rac1/Rac2 from T-cell lineage significantly inhibited TCR cross-linking–stimulated cell growth. We further observed a significant decrease of IL-2 production in the culture medium of Rac1/Rac2-deificent thymocytes (Figure 7B), and the growth defect of Rac1/Rac2-deficient thymocytes could be partially rescued by the addition of exogenous IL-2 (Figure 7A). These data suggest that Rac1/Rac2 regulate thymocyte T-cell growth partly by modulating IL-2 production. Similarly, splenic T cells from Rac1/Rac2-deficient mice also displayed a defect in TCR activation-induced cell growth (Figure 7C).
T cell–specific deletion of Rac1/Rac2 inhibits TCR-induced cell growth and signaling. Single-cell suspensions of thymocytes or splenic T cells were prepared from mice of the indicated genotypes. The cells were plated on 96-well plates at 2 × 105/well in 100 μL culture with or without anti-TCR antibody or IL-2 (A-C). The medium was collected at day 2 from the thymocyte culture for IL-2 assay by ELISA (B), and the cell numbers were counted after a 3-day culture (A,C). Data are expressed as the fold of growth relative to the number of WT cells without stimulation. Error bars represent the standard deviations of 5 WT and 5 Rac1−/−Rac2−/− mice. *P < .05; **P < .01. (D) Western blotting was performed to assess the phosphorylation status of Akt, Erk, p38, and ZAP70 after a 3-minute TCR cross-linking of freshly isolated thymocytes. The quantitative results were shown as ratios between normalized phospho-Akt, -Erk, -p38, or -ZAP70 and total Akt, Erk, p38, or ZAP70, and are presented as means plus or minus SD from 5 WT and 5 Rac1−/−Rac2−/− mice. Rac1 expression was probed by anti-Rac1 blotting in parallel. Error bars represent SD.
T cell–specific deletion of Rac1/Rac2 inhibits TCR-induced cell growth and signaling. Single-cell suspensions of thymocytes or splenic T cells were prepared from mice of the indicated genotypes. The cells were plated on 96-well plates at 2 × 105/well in 100 μL culture with or without anti-TCR antibody or IL-2 (A-C). The medium was collected at day 2 from the thymocyte culture for IL-2 assay by ELISA (B), and the cell numbers were counted after a 3-day culture (A,C). Data are expressed as the fold of growth relative to the number of WT cells without stimulation. Error bars represent the standard deviations of 5 WT and 5 Rac1−/−Rac2−/− mice. *P < .05; **P < .01. (D) Western blotting was performed to assess the phosphorylation status of Akt, Erk, p38, and ZAP70 after a 3-minute TCR cross-linking of freshly isolated thymocytes. The quantitative results were shown as ratios between normalized phospho-Akt, -Erk, -p38, or -ZAP70 and total Akt, Erk, p38, or ZAP70, and are presented as means plus or minus SD from 5 WT and 5 Rac1−/−Rac2−/− mice. Rac1 expression was probed by anti-Rac1 blotting in parallel. Error bars represent SD.
Rac1 and Rac2 have been shown to regulate Erk and p38 MAP kinases and Akt activity in various cell lineages. These signaling molecules contribute to T-cell development by mediating cell proliferation and/or survival.38 We investigated the activities of Erk, p38, Akt, and ZAP70, another essential regulator of T-cell development, in WT and Rac1/Rac2-deficient thymocytes. The basal level of Akt activity in Rac1/Rac2-deficient thymocytes is low and statistically indistinguishable from that in WT cells. The Akt activity in WT cells was significantly up-regulated following TCR cross-linking. However, the Akt activity in activated Rac1/Rac2-deficient thymocytes was drastically less than that in control WT cells, suggesting that Akt plays a role in Rac1- and Rac2-regulated T-cell development. Unexpectedly, TCR cross-linking was able to activate p38, Erk, and ZAP70 to a similar extent in WT and Rac1/Rac2-deficient thymocytes (Figure 7D), suggesting that Rac1/Rac2 do not participate in the regulation of these signaling kinases, at least in the context of TCR activation.
Discussion
In the current study, we show by one mouse gene targeting approach (Mx-Cre) that both Rac1 and Rac2 are required for proper T-lineage progenitor, CLP, production in the bone marrow. By another T cell–specific gene targeting model (Lck-Cre), we show that whereas depletion of Rac1 alone has a limited effect on T-cell maturation in the thymus, deletion of both Rac1 and Rac2 severely inhibits T cell development and the ability of mature T cells to populate the peripheral spleen and blood. Since Rac2-deficient mice were found to be mostly normal in thymocyte development (Table S1; Croker et al25 ), our results suggest that Rac1 and Rac2 play redundant, but critical, roles in T-cell development. Further, our data implicate that a combined function of Rac1/Rac2 in cell proliferation, survival, adhesion, and migration is involved in the regulation of T-cell maturation.
The role of Rac GTPases in T lymphocyte functions has been explored previously in T-cell lines by transfection of dominant active or negative mutant of Rac1. It has been observed that overexpression of a constitutively active mutant of Rac1 (Rac1V12) triggered dramatic spreading and increased adhesion of Jurkat T cells on fibronectin in an integrin-dependent manner.14 Transfection of T-cell lines that constitutively display a polarized motile morphology with Rac1V12 impaired cell polarity, whereas dominant negative form of Rac1 (Rac1N17) induced a polarized phenotype in constitutively round-shaped T cells. Moreover, overexpression of Rac1V12 significantly inhibited SDF-1α–induced T-cell chemotaxis.15 Although these conventional studies are informative for certain T-cell regulatory roles by Rac, they lack functional specificity and do not provide insight into the potential functions of individual Rac GTPases in T-cell development in vivo.
We have explored the individual and combined role of Rac1 and Rac2 GTPases in thymopoiesis in HSC-specific and T-lineage–specific mouse genetic models. Although consistently revealing a redundant but essential role of Rac1 and Rac2 in T-cell development, these 2 approaches are complementary in several aspects. First, deletion of Rac1 from Rac1loxp/loxp mice by Mx-Cre impairs, albeit modestly, T-cell development in thymus, and causes a decrease in mature T-cell number in peripheral blood and spleen. In contrast, deletion of Rac1 from Rac1loxp/loxp mice by Lck-Cre shows no significant effect on T-cell differentiation in thymus and on mature T-cell production in peripheral blood and spleen. Second, deletion of Rac1 from Rac1loxp/loxp/Rac2−/− mice by Mx-Cre alters the numbers of CD4−CD8− DN and CD8+ SP thymocytes, whereas deletion of Rac1 from Rac1loxp/loxp/Rac2−/− mice by Lck-Cre has no effect on these 2 subpopulations. Third, deletion of Rac1 from Rac1loxp/loxp/Rac2−/− mice by Mx-Cre increases the proportion of apoptotic CD8+ SP thymocytes, whereas deletion of Rac1 from Rac1loxp/loxp/Rac2−/− mice by Lck-Cre shows no effect on the survival capability of CD8+ SP thymocytes. These differences in the 2 mouse model systems could be attributed to several factors: (1) Although the time lapsed from poly I:C administration to T-cell characterization was usually more than 15 days and the control WT mice were subjected to similar treatment, we cannot rule out a potential residual IFN effect induced by poly I:C in the knockout mice that may affect T-cell survival. (2) Mx-Cre–mediated deletion of Rac1 leads to a defect in CLP differentiation in bone marrow, which may have lingering effect on later stages of T-cell development in thymus. (3) Mx-Cre deletes Rac1 from all HSC-derived blood cell lineages including antigen-presenting cells, which may affect the microenvironment of T-cell development and survival. These considerations led us to a more careful examination of Rac1/Rac2 function by T-lineage–specific, Lck-Cre–mediated recombination. The limitations imposed by using the Lck-Cre system, including the relatively delayed deletion of Rac1 gene during T-cell development, on the other hand, were partly complemented by the Mx-Cre mouse model.
By comparing Rac1 with Rac2, both of which serve critical but overlapping roles in B-cell development,31 it appears that Rac1 and Rac2 also play overlapping and essential roles in T lymphopoiesis. It is noted that deletion of Rac1 or Rac1/Rac2 at the stem cell stage inhibits CLP production. However, the number of CLP-derived B cells in peripheral blood increases, rather than decreases, by the loss of Rac1 or Rac1/Rac2. This apparent paradox could be explained by the potential hyperproliferative nature of the post-CLP developmental stages of B cells. It is also worth noting that the elevated B-cell numbers in the Rac1/Rac2 double knockout mice are likely attributable to the loss of Rac2 and may not be related to Rac1 loss, since previous characterizations of Rac2 knockout mice have documented the increased B-cell phenotype.36
Cell proliferation, survival, adhesion, and migration play pivotal roles in T-cell development.16,39 In agreement with this, we observed that the altered cell proliferation, survival, adhesion, and/or migration by deletion of Rac1/Rac2 are associated with, and likely contribute to, the reduction of the thymocyte and splenocyte T-cell subpopulations. To further understand the mechanisms underlying Rac1/Rac2-mediated T-cell development, we have examined the expression level of CD69, which is up-regulated during positive selection, in CD4+CD8+ DP thymocytes. A significant decrease of CD69 expression in Rac1/Rac2-deficient DP cells, compared with that in WT cells, was observed, indicating a defective positive selection in Rac1/Rac2-null mice. Moreover, we have investigated thymocyte and splenocyte proliferative potential of Rac1/Rac2-deficient mice. We found that disruption of Rac1/Rac2 impairs proliferation of thymocytes and splenocytes in response to TCR activation, an effect associated with defective Akt signaling response, not the Erk or p38 MAP kinase responses that have been shown to be involved in positive and negative selection and in cell proliferation and survival.37 IL2 production was also significantly dampened in Rac1/Rac2-deficient thymocytes, indicating IL2 regulation by Rac1/Rac2 contributes to the growth signaling. These mechanistic characterizations suggest that Akt signaling and IL2 production are important in Rac1- and Rac2-regulated T-cell development and function. Further work is warranted to definitively define the signaling pathways regulated by Rac1/Rac2 in the various stages of T-cell development as revealed in the present studies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by NIH grants R01 CA125658, R01 GM60523, and R01 DK62757, and by American Heart Association (Washington, DC) Beginning-Grant-In-Aid 0765194B.
National Institutes of Health
Authorship
Contribution: F.G. and Y.Z. performed research, contributed vital new reagents or analytical tools, analyzed data, and wrote the paper; J.A.C. and D.H. performed research and analyzed data; and D.A.W. analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yi Zheng, 3333 Burnet Avenue, Cincinnati, OH 45229; e-mail: yi.zheng@cchmc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal