Abstract
Low-level expression of multiple lineage-specific genes is a hallmark of hematopoietic stem cells (HSCs). HSCs predominantly express genes specific for the myeloid or megakaryocytic-erythroid lineages, whereas the transcription of lymphoid specific genes appears to begin after lymphoid specification. It has been demonstrated for a number of genes that epigenetic priming occurs before gene expression and lineage specification; however, little is known about how epigenetic priming of lymphoid genes is regulated. To address the question of how B cell–restricted expression is established, we studied activation of the Cd19 gene during hematopoietic development. We identified a B cell–specific upstream enhancer and showed that the developmental regulation of Cd19 expression involves precisely coordinated alterations in transcription factor binding and chromatin remodeling at Cd19 cis-regulatory elements. In multipotent progenitor cells, Cd19 chromatin is first remodeled at the upstream enhancer, and this remodeling is associated with binding of E2A. This is followed by the binding of EBF and PAX5 during B-cell differentiation. The Cd19 promoter is transcriptionally activated only after PAX5 binding. Our experiments give important mechanistic insights into how widely expressed and B lineage–specific transcription factors cooperate to mediate the developmental regulation of lymphoid genes during hematopoiesis.
Introduction
It has previously been shown that hematopoietic stem cells (HSCs) express a number of granulocyte-macrophage (GM) and megakaryocytic-erythroid (MkE) associated genes at low levels,1,2 and a recent study showed that this lineage priming occurs in a hierarchical fashion.3 In contrast to GM- and MkE-associated genes, the majority of lymphoid specific genes, including Cd19, appear to be expressed only in cells already specified as lymphoid. This was confirmed by a highly sensitive lineage-tracing technique.4 Using mice carrying a linegate affiliating marker, it was demonstrated that the myelomonocyte-specific Lysozyme M gene, but not Cd19, is expressed in long-term reconstituting HSCs. The chromatin structure of some B cell–specific genes is already partially reorganized before transcription. One of the pre-B cell–receptor (BCR) components, immunoglobulin lambda-like polypeptide 1 (Igll1), is expressed exclusively in pro- and pre-B cells. In an early B-cell progenitor cell line (Ba/F3), the upstream enhancer of this gene is DNaseI hypersensitive, enriched with histones carrying active modifications and binds general transcription factors (GTF) as well as RNA polymerase II (RNAP II) even before detection of an mRNA transcript.5 It is, however, not yet known how B lymphoid– specific gene loci are epigenetically regulated when cells transit from multipotent progenitor cells to committed B cells
B-cell differentiation involves a number of transcription factors that participate in gene activation and repression at several stages in B-cell differentiation. Among them, E2A, EBF, and PAX5 are essential for B-lineage specification, commitment, and maintenance. In the absence of either E2A or EBF, B-cell differentiation is arrested at the pre-pro-B stage where immunoglobulin DH-JH rearrangement has not yet occurred.6-8 In the absence of PAX5, differentiation arrest occurs at a later stage (early pro-B).9 E2A, EBF, and PAX5 regulate each other and cooperate to activate a B-cell specific gene expression profile. For example, E2A induces EBF expression through binding to the EBF promoter,10,11 EBF up-regulates PAX5, and vice versa. The promoter of the signal transduction molecule, Cd79a, is regulated cooperatively by E2A, EBF, and PAX5 as well as RUNX1,12,13 and E2A and EBF can synergistically activate the immunoglobulin surrogate light chain (Vpreb1 and Igll1).14,15 Although it is thought that E2A acts upstream of EBF and PAX5, it is not yet known whether these proteins act simultaneously or successively at specific cis-regulatory elements of their target.
To gain insight into how lineage priming and specification in early B-cell differentiation takes place at the epigenetic level, we examined the regulation of the B-cell specific gene Cd19. CD19 is a signal transduction molecule participating in BCR and pre-BCR signaling. At the early stage of B-cell differentiation, signaling through the pre-BCR, composed of IgH, VpreB, and λ5, drives the cells to complete immunoglobulin light chain rearrangement, to down-regulate Vpreb1 and Igll1, and to transit from pre-B cells to immature B cells, which express the BCR on their surface. Thereafter, the maturation, proliferation, and activation of B lymphocytes are dependent on BCR signals (reviewed by Del Nagro et al16 ). CD19 is part of a multiprotein complex that positively regulates both BCR and pre-BCR signals. The importance of CD19 in vivo was demonstrated by Cd19 knockout mice, which have less serum immunoglobulin, fewer B cells, and a reduction of germinal center formation in response to T cell–dependent antigens.17-19 The correct level of Cd19 expression is also important because overexpression leads to a suppression of immature B-cell development.20 With a few exceptions, such as bipotential B-macrophage progenitors21 and a small population of dendritic cells,22 the expression of CD19 is strictly restricted to B lymphocytes.
The molecular mechanisms that regulate tissue and developmental stage–specific expression of Cd19 are poorly understood. It has been shown that the B cell–specific transcription factor PAX5 binds to the human CD19 promoter23 ; and in the absence of PAX5, pro-B cells fail to express Cd19. Expression can be restored by the introduction of PAX5 without de novo protein synthesis.24 Transgenes containing human CD19 with either 6.3 kb or 4.5 kb 5′ flanking regions, were expressed in a B cell–specific manner,20,25 whereas transgenes with the promoter were expressed weakly in B cells and other hematopoietic cells,26,27 indicating additional mechanisms restricting and activating Cd19 expression in B lymphocytes.
To examine the molecular mechanisms that are involved in lineage-specific Cd19 activation in B-cell differentiation, we studied the chromatin configuration of the mouse Cd19 locus during hematopoiesis. Using DNaseI hypersensitive site (DHS) mapping and transfection studies, we identified a B cell–specific enhancer. Chromatin remodeling at the promoter as well as the transcription initiation was strictly dependent on the presence of PAX5. In contrast, chromatin was remodeled in the absence of PAX5 and was already epigenetically primed in unspecified multipotent progenitors at the enhancer. This early priming was associated with the binding of E2A and was followed by the binding of EBF and then PAX5 during B-cell differentiation. Our experiments show that before B-cell commitment the Cd19 locus is primed at the level of chromatin and activated by the collaboration of transcription factors, which are sequentially recruited to its regulatory region.
Methods
The biologic safety, including radiation safety and genetic modification procedure, was reviewed by the University Health and Safety Committee within the University of Leeds. All procedures involving experimental animals were handled under the regulation of Animals (Scientific Procedures) Act 1986 and reviewed by the Home Office in the United Kingdom and the University of Leeds.
Antibodies
Chromatin immunoprecipitation assays.
Chromatin immunoprecipitation (ChIP) assays included RNAP II (sc-900X; Santa Cruz Biotechnology, Santa Cruz, CA), H3K4me1 (ab-8895; Abcam, Cambridge, United Kingdom), H3K4me3 (ab-8580; Abcam), H3K9me1 (ab-9045; Abcam), PAX5 (rabbit polyclonal antibody from M. Busslinger, sc-1975X; Santa Cruz Biotechnology), E2A (rabbit polyclonal Ab from M. Busslinger), and EBF (rabbit polyclonal antibody from M. Busslinger).
Cell purification.
Cell purification included fluorescein isothiocyanate-anti–c-Kit, phycoerythrin (PE)–cyanin 7–anti-CD19, allophycocyanin–anti-B220, PE–anti-CD43, PE–anti-IgD, PE-Cy5-streptavdin (BD Biosciences, San Jose, CA), PE–anti-IL-7R, PE-Cy7-anti–Sca-1, biotin–anti-BP-1, fluorescein isothiocyanate–anti-IgM (eBioscience, San Diego, CA), biotin-ER-MP12 (BMA Biomedicals, Augst, Switzerland), anti-B220 (RA3-3A1 from V. Tybulewitz), and anti–I-A/I-E (M5/114 from V. Tybulewicz, National Institute for Medical Research, London, United Kingdom).
CpG methylation analysis
Genomic DNA (0.2-1 μg) was subjected to sodium bisulfite treatment and purified using the EZ DNA Methylation Kit (Zymo Research, Orange, CA). Purified DNA was amplified by polymerase chain reaction (PCR) using primers specific to the modified sequence. PCR amplified DNA fragments were directly sequenced using the Thermo Sequenase Cycle Sequencing Kit (USB, Cleveland, OH), and band intensities corresponding to T and C were quantified using the PharosFX Plus system (Bio-Rad, Hercules, CA) and Quantity One software. The level of modification was determined as [intensity (C)/(intensity (T) + intensity (C))].
DHS mapping
DNaseI treatment was performed as previously described.28 In summary, cells were treated with various concentrations of DNaseI (Worthington Biochemicals, Lakewood, NJ) in ψ buffer containing 0.2% Nonidet P-40 for 7 minutes at 20°C; 10 μg DNA for each sample was digested with EcoRI, BlpI, or ScaI to completion and subjected to Southern blot analysis. The positions of the restriction enzyme sites and probes are described in Figure S1A (available on the Blood website; see the Supplemental Materials link at the top of the online article).
ChIP
For RNAP II and histone modification, ChIP assay was performed as previously described.29 Briefly, chromatin was prepared after cross-linking with 1% formaldehyde for 10 minutes at room temperature and sheared in 0.1% sodium dodecyl sulfate using a Sonifier homogenizer. Each precipitation was performed using chromatin from 107 cells, 1 μg antibody, and 20 μL protein A agarose (Thermo Fisher Scientific, Rockford, IL). To precipitate transcription factor bound chromatin, the chromatin was prepared in the same way but sheared in buffer containing 0.2% sodium dodecyl sulfate using a Bioruptor sonicating water bath. The chromatin from 2 × 106 cells, 1 μg antibody, and 10 μL Dynabeads protein G were used for each precipitation.
Precipitated DNA was quantified using real time quantitative PCR with SYBR Green. Primers used in this assay are listed in Table S1.
Cell purification
Progenitors (LSKs, CMPs, and CLPs) and B lymphocytes were purified by cell sorting using a FACS Vantage (BD Biosciences, San Jose, CA) or MoFlo (Beckman Coulter, Fullerton, CA) from mouse bone marrow after lineage depletion using antibodies against myeloid cells (Gr-1, Ter119, CD11b, F4/80, and ER-MP20 (Ly-6C)) and T cells (CD3, CD4, and CD8). The cell-sorting profile is described in Figure S3. Splenic B cells were obtained by depleting myeloid and T-cell lineage cells from spleen cells by staining with Gr-1, Ter119, CD11b, ER-MP20 (Ly-6C), and thy1.2 antibodies followed by complement-dependent cell lysis and purification using Low-Tox-M Rabbit complements and Lympholyte-M (Cedarlane Laboratories, Burlington, ON). For thymic and splenic T cells, major histocompatibility complex–positive cells (I-A/I-E: M5/114) and B lineage (B220: RA3–3A1) were depleted from thymus and spleen, respectively.
In vitro cell culture
Bone marrow-derived macrophages and mouse embryonic fibroblasts were prepared as described.30 Wild-type, Pax5−/−, and Pax5-ER cells were cultured as described previously.29 Mouse B-cell lines (38B9, 300-19, Bal17, A20) were cultured in RPMI 1640 supplemented with antibiotics and 10% fetal calf serum (FCS), EL4 in Dulbecco minimal essential medium (DMEM) with antibiotics and 5% horse serum, and RAW264 and 3T3 in DMEM with antibiotic and 10% FCS. ES cells were maintained in DMEM supplemented with 15% FCS, 0.15 mM monothioglycerol, 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer, 2 mM glutamine, antibiotics, and 0.7% leukemia inhibitory factor containing conditioned medium in the presence of mitomycin C-treated mouse embryonic fibroblasts (MEFs) and expanded in the same media without MEFs in gelatin-coated flasks.
Gene expression analysis
In vivo footprinting
Transient transfection assays
All reporter gene constructs in this study were derived from pXPG.31 Cd19 promoter sequences (from −7 to −200) only or promoter plus enhancer (−1832 to −2096) were inserted upstream of the luciferase gene. Transient transfections of RAW264 cells (106 cells per transfection) and 3T3 (105 cells per transfection) were performed in triplicate using jetPEI transfection reagent (Polyplus-transfection, Strasbourg, France) exactly as described previously.29 Transfection of suspension cells was performed by electroporation (Gene Pulser II; Bio-Rad); 107 cells in 0.4 mL growth media were used for each transfection; 10 μg reporter constructs was transfected with 20 ng pRLCMV (Promega, Madison, WI). Luciferase activity was measured using Dual-Glo Luciferase Assay System (Promega).
Relative activity (Figure 1) was determined as the luciferase activity of the reporter construct divided by that of the control, pXPG. For these assays, variation in the assay was corrected by normalizing to Renilla luciferase activity.
Results
Identification of a B cell–specific upstream enhancer at the Cd19 locus
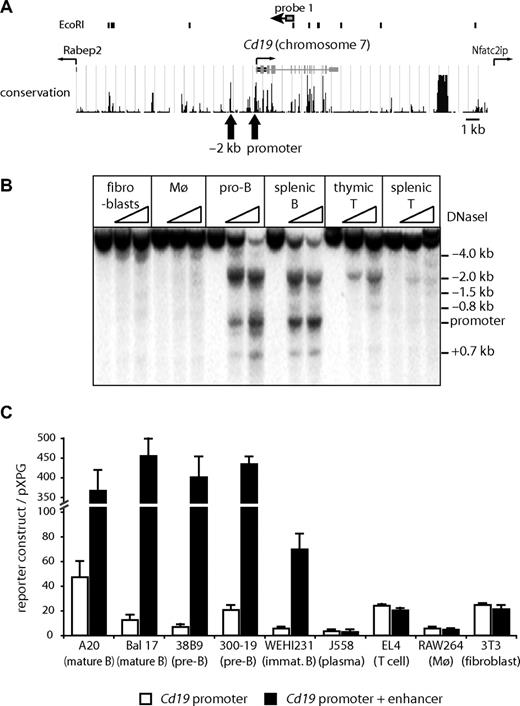
Cd19 occupies only 32 kb between 2 neighboring genes (Figure 1A). To investigate whether there were additional cis-regulatory elements within the Cd19 locus, we first mapped DHSs, which are indicative of active cis-regulatory elements. Among the cell types tested, the promoter was strongly DNaseI hypersensitive only in B cells. We found at least 8 ubiquitous and 4 hematopoietic specific DHSs (Figure S1B), most of which were weak except for a highly conserved strong DHS approximately 2 kb upstream of the transcription start sites. This DHS was strong in B cells, weaker in thymic T cells, and even weaker in mature T cells (Figure 1B) but was not present in macrophages and fibroblasts. We confirmed that the −2 kb DHS is a functional enhancer element by performing transient transfection experiments (Figure 1C). As previously reported in human cells, the promoter was active in all analyzed cell types (Figure 1C white bars). In contrast, the −2 kb DHS had enhancer activity only in B cells (Figure 1C black bars), providing evidence that this enhancer is involved in the regulation of tissue specific expression of Cd19.
The DNaseI hypersensitive site at −2 kb has B cell–specific enhancer activity. (A) Map of the mouse Cd19 locus on chromosome 7. (Top panel) Position of EcoRI sites and probe 1 used in Southern blotting analysis. (Middle panel) Position of introns, exons, transcription start of Cd19, and neighboring genes and conservation between different species (http://genome.ucsc.edu/).  indicates the position of DHS corresponding to the promoter and the enhancer. (B) DNaseI hypersensitive site mapping assays demonstrating strong hypersensitive sites at −2 kb and the promoter. Genomic DNA from DNaseI-treated cells was digested with EcoRI and subjected to Southern blot analysis using probe 1. Cells used were mouse embryonic fibroblast (fibroblasts), bone marrow-derived macrophages (Mø), pro-B cells (pro-B), CD19+ splenocytes (splenic B), Thy1+ thymocytes (thymic T), and Thy1+ splenocytes (splenic T). (C) Transient transfection assays in different mouse cell lines. A mouse Cd19 promoter reporter construct (−7 to −200 bp; □) or a construct containing the −2 kb DHS (−1832 to −2096 bp) combined with the Cd19 promoter (■) was transiently transfected into various cell lines. The relative activity was determined as the luciferase activity of each construct over control vector pXPG. Data represent the mean of 2 to 4 experiments performed in triplicate.
indicates the position of DHS corresponding to the promoter and the enhancer. (B) DNaseI hypersensitive site mapping assays demonstrating strong hypersensitive sites at −2 kb and the promoter. Genomic DNA from DNaseI-treated cells was digested with EcoRI and subjected to Southern blot analysis using probe 1. Cells used were mouse embryonic fibroblast (fibroblasts), bone marrow-derived macrophages (Mø), pro-B cells (pro-B), CD19+ splenocytes (splenic B), Thy1+ thymocytes (thymic T), and Thy1+ splenocytes (splenic T). (C) Transient transfection assays in different mouse cell lines. A mouse Cd19 promoter reporter construct (−7 to −200 bp; □) or a construct containing the −2 kb DHS (−1832 to −2096 bp) combined with the Cd19 promoter (■) was transiently transfected into various cell lines. The relative activity was determined as the luciferase activity of each construct over control vector pXPG. Data represent the mean of 2 to 4 experiments performed in triplicate.
The DNaseI hypersensitive site at −2 kb has B cell–specific enhancer activity. (A) Map of the mouse Cd19 locus on chromosome 7. (Top panel) Position of EcoRI sites and probe 1 used in Southern blotting analysis. (Middle panel) Position of introns, exons, transcription start of Cd19, and neighboring genes and conservation between different species (http://genome.ucsc.edu/).  indicates the position of DHS corresponding to the promoter and the enhancer. (B) DNaseI hypersensitive site mapping assays demonstrating strong hypersensitive sites at −2 kb and the promoter. Genomic DNA from DNaseI-treated cells was digested with EcoRI and subjected to Southern blot analysis using probe 1. Cells used were mouse embryonic fibroblast (fibroblasts), bone marrow-derived macrophages (Mø), pro-B cells (pro-B), CD19+ splenocytes (splenic B), Thy1+ thymocytes (thymic T), and Thy1+ splenocytes (splenic T). (C) Transient transfection assays in different mouse cell lines. A mouse Cd19 promoter reporter construct (−7 to −200 bp; □) or a construct containing the −2 kb DHS (−1832 to −2096 bp) combined with the Cd19 promoter (■) was transiently transfected into various cell lines. The relative activity was determined as the luciferase activity of each construct over control vector pXPG. Data represent the mean of 2 to 4 experiments performed in triplicate.
indicates the position of DHS corresponding to the promoter and the enhancer. (B) DNaseI hypersensitive site mapping assays demonstrating strong hypersensitive sites at −2 kb and the promoter. Genomic DNA from DNaseI-treated cells was digested with EcoRI and subjected to Southern blot analysis using probe 1. Cells used were mouse embryonic fibroblast (fibroblasts), bone marrow-derived macrophages (Mø), pro-B cells (pro-B), CD19+ splenocytes (splenic B), Thy1+ thymocytes (thymic T), and Thy1+ splenocytes (splenic T). (C) Transient transfection assays in different mouse cell lines. A mouse Cd19 promoter reporter construct (−7 to −200 bp; □) or a construct containing the −2 kb DHS (−1832 to −2096 bp) combined with the Cd19 promoter (■) was transiently transfected into various cell lines. The relative activity was determined as the luciferase activity of each construct over control vector pXPG. Data represent the mean of 2 to 4 experiments performed in triplicate.
The Cd19 promoter binds PAX5, whereas the enhancer binds E2A, EBF, and PAX5
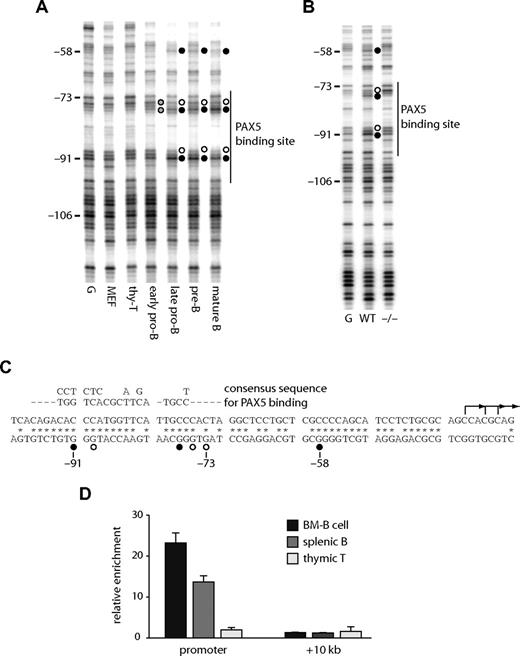
We performed an in vivo DMS footprinting assay to answer the question of which transcription factors bind to the Cd19 promoter and enhancer. A high affinity PAX5 binding site (Figure 2C) at the promoter is known to bind PAX5 in human B cells.23 Figure 2A shows that this binding site was occupied in all Cd19-expressing mouse cells. Weak footprints (indicated as gray circles) were observed at the early pro-B cell stage and became stronger when the cells transited to the late pro-B stage. These footprints were observed in wild-type but not in Pax5−/− pro-B cells (Figure 2B), further demonstrating that they are the result of PAX5 binding. The binding of PAX5 at the promoter in primary cells was confirmed by performing a ChIP assay with purified B cells (Figure 2D).
The Cd19 promoter binds PAX5 in B cells in vivo. DMS in vivo footprinting assays were performed at the Cd19 promoter in purified primary cells (A) and pro-B cell lines (B). Early pro-B, late pro-B, pre-B, and mature B cells were purified from bone marrow (Figure S3). G indicates in vitro DMS-treated DNA; MEF, mouse embryonic fibroblast; thy-T, THY1+ thymocytes; WT, wild-type pro-B cells; −/−, Pax5− pro-B cells. Numbers on the left indicate the positions relative to the ATG. A vertical line on the right is indicative of a high affinity PAX5 binding site. ● and ○ represent guanine residues hyperreactive or hyporeactive to DMS, respectively; gray circles, partial footprints. (C) DNA sequence of the mouse Cd19 promoter. * Nucleotide sequences homologous to the human CD19 locus. ● and ○ represent nucleotides occupied by transcription factors as assayed by in vivo footprinting. The consensus PAX5 recognition sequence is aligned above the promoter sequence. L-shaped arrows are major transcription start sites as determined by reverse transcription transferase-dependent PCR (RT-TDPCR; data not shown). (D) Recruitment of PAX5 in primary cells. CD19+ bone marrow cells (BM-B), CD19+ spleenocytes (splenic B), and Thy-1+ thymocytes (thymic T) cells were purified, crosslinked, and subjected to ChIP assay using a polyclonal antibody against PAX5. Precipitated DNA was amplified with primers specific for the Cd19 promoter, +10 kb regions, and 45S rRna promoter50 (control). Each bar represents the relative enrichment compared with the control region.
The Cd19 promoter binds PAX5 in B cells in vivo. DMS in vivo footprinting assays were performed at the Cd19 promoter in purified primary cells (A) and pro-B cell lines (B). Early pro-B, late pro-B, pre-B, and mature B cells were purified from bone marrow (Figure S3). G indicates in vitro DMS-treated DNA; MEF, mouse embryonic fibroblast; thy-T, THY1+ thymocytes; WT, wild-type pro-B cells; −/−, Pax5− pro-B cells. Numbers on the left indicate the positions relative to the ATG. A vertical line on the right is indicative of a high affinity PAX5 binding site. ● and ○ represent guanine residues hyperreactive or hyporeactive to DMS, respectively; gray circles, partial footprints. (C) DNA sequence of the mouse Cd19 promoter. * Nucleotide sequences homologous to the human CD19 locus. ● and ○ represent nucleotides occupied by transcription factors as assayed by in vivo footprinting. The consensus PAX5 recognition sequence is aligned above the promoter sequence. L-shaped arrows are major transcription start sites as determined by reverse transcription transferase-dependent PCR (RT-TDPCR; data not shown). (D) Recruitment of PAX5 in primary cells. CD19+ bone marrow cells (BM-B), CD19+ spleenocytes (splenic B), and Thy-1+ thymocytes (thymic T) cells were purified, crosslinked, and subjected to ChIP assay using a polyclonal antibody against PAX5. Precipitated DNA was amplified with primers specific for the Cd19 promoter, +10 kb regions, and 45S rRna promoter50 (control). Each bar represents the relative enrichment compared with the control region.
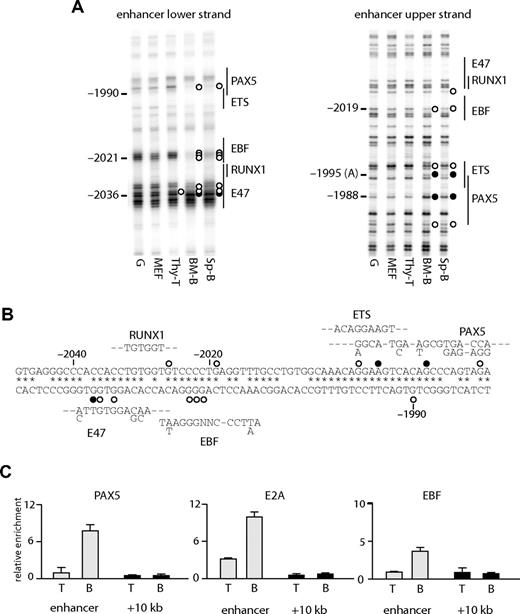
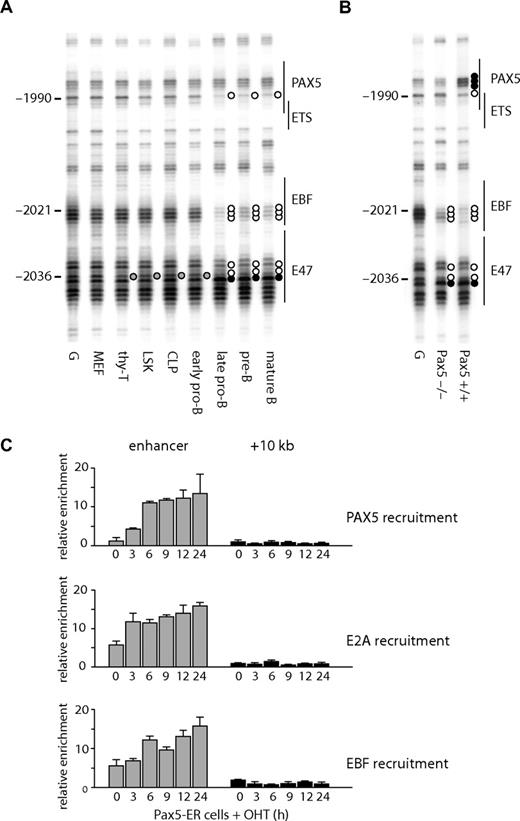
At the enhancer, several B cell–specific footprints were observed. These included a footprint over a putative EBF binding site (observed as a decreased modification by DMS at −2021 to −2023 on the lower strand and −2019 on the upper strand) and at a PAX5 binding site (hypo-methylation at −1990 on the lower strand, at −1980 on the upper strand, and hyper-methylation at −1988 on the upper strand). A hypo-methylated guanine at a putative E47 binding site (−2036) was observed in T cells, as well as B cells, but not in fibroblasts (Figure 3A,B). The binding of E2A at this region may be responsible for the weak hypersensitivity in T cells (Figure 1B). The specific binding of these proteins was confirmed by ChIP analysis (Figure 3C), demonstrating that EBF and PAX5 bound to the enhancer in B cells and that E2A bound in B and T cells. We also observed footprints at a putative ETS binding site (Figure 3A right panel), although we have not yet identified which factor binds to this sequence.
Transcription factor assembly at the Cd19 enhancer. (A) In vivo DMS footprinting assays were performed using primary cells. DMS-modified DNA was amplified using LM-PCR with primers specific for the upper strand of the enhancer region. G indicates in vitro DMS treated DNA; MEF, mouse embryonic fibroblast; thy-T, Thy1+ thymocytes; BM-B, CD19+ bone marrow cells; Sp-B, CD19+ splenocytes. Vertical lines on the right indicate transcription factor binding sites identified by sequence analysis. Symbols are as in Figure 2. (B) Sequence alignment with consensus binding sites. DNA sequence at the enhancer was aligned with consensus sequence of transcription factor binding sites (E47, EBF, RUNX1, ETS, PAX5). Symbols are as in Figure 2. (C) ChIP experiments performed on Thy1+ thymocytes (T) and CD19+ splenocytes (B) using antibodies against PAX5, E2A, and EBF. Enrichment was measured at the enhancer and a control region at +10 kb. Relative enrichment was normalized against 45S rRna promoter.
Transcription factor assembly at the Cd19 enhancer. (A) In vivo DMS footprinting assays were performed using primary cells. DMS-modified DNA was amplified using LM-PCR with primers specific for the upper strand of the enhancer region. G indicates in vitro DMS treated DNA; MEF, mouse embryonic fibroblast; thy-T, Thy1+ thymocytes; BM-B, CD19+ bone marrow cells; Sp-B, CD19+ splenocytes. Vertical lines on the right indicate transcription factor binding sites identified by sequence analysis. Symbols are as in Figure 2. (B) Sequence alignment with consensus binding sites. DNA sequence at the enhancer was aligned with consensus sequence of transcription factor binding sites (E47, EBF, RUNX1, ETS, PAX5). Symbols are as in Figure 2. (C) ChIP experiments performed on Thy1+ thymocytes (T) and CD19+ splenocytes (B) using antibodies against PAX5, E2A, and EBF. Enrichment was measured at the enhancer and a control region at +10 kb. Relative enrichment was normalized against 45S rRna promoter.
The chromatin at the −2 kb enhancer is remodeled before the expression of PAX5, but activation of the promoter requires PAX5
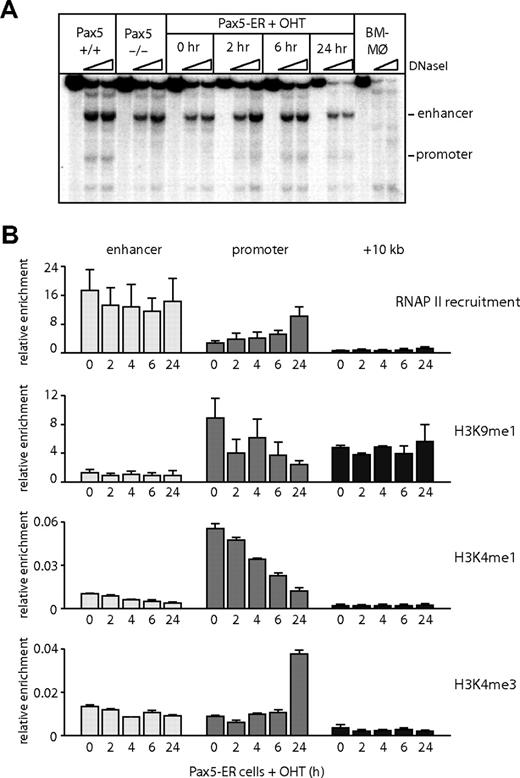
Although the −2 kb enhancer was active in a B cell–specific fashion in transient transfection assays, DNaseI hypersensitivity was also detected in early T cells and, to a lesser extent, later in T-cell development. We therefore hypothesized that the enhancer is remodeled at an early stage of hematopoietic development before B-cell commitment and is then progressively silenced in T cells. To test this hypothesis, we took advantage of a pro-B cell line derived from Pax5−/− animals into which an estrogen-inducible PAX5 had been reintroduced (Pax5-ER cells24 ). It has been demonstrated that Pax5−/− cells can differentiate into multiple hematopoietic lineages with the exception of B cells and thus resemble early multipotent progenitor cells.32,33 After induction of the active form of PAX5 protein by 4-hydroxy-tamoxifen (OHT), it was recruited to the promoter (Figure S2B,C). The binding of RNAP II, the acquisition of DNaseI hypersensitivity at the Cd19 promoter, and Cd19 mRNA expression required the induction of PAX5 (Figures 4A,B, S2A).24,29 However, even in the absence of PAX5, chromatin was in a poised configuration, as indicated by the presence of monomethylated histone H3 lysine 9 (H3K9me1) and histone H3 lysine 4 (H3K4me1).34,35 The level of these modifications was down-regulated in a PAX5-dependent fashion, and we observed a transition from monomethylated to trimethylated H3K4, confirming that the RNAP II recruited to the promoter was actively transcribing. In contrast to the promoter, the enhancer was hypersensitive to DNaseI and showed a low level of H3K9me1 in the absence of PAX5 (Figure 4A,B), indicating that the chromatin at the enhancer was already remodeled in these multipotent cells. We also observed a constitutive recruitment of RNAP II at the enhancer (Figure 4B); however, the level of histone H3K4me3 was relatively low (Figure 4B), indicating that the RNAP II recruited to the enhancer is not transcribing at a high rate. In summary, these data show that activation of the Cd19 locus during B lymphopoiesis starts at the upstream enhancer, but onset of transcription is determined by the successive activation of the promoter by PAX5.
The Cd19 enhancer is reorganized and accessible before PAX5 expression. (A) DNaseI hypersensitivity assays were performed in bone marrow–derived macrophages (BM-Mø), wild-type (Pax5+/+), and Pax5−/− pro-B cells and Pax5-ER cells induced with OHT for 0, 2, 6, and 24 hours. DNaseI-treated DNA was analyzed by Southern blot as described in Figure 1. (B) RNAP II recruitment, the levels of monomethylated histone H3 lysine 9 (H3K9), monomethylated, and trimethylated histone H3 lysine 4 (H3K4) were measured by ChIP assays in Pax5-ER cells induced with OHT. The enrichment was measured at the promoter, enhancer, and a downstream region at +10 kb. The values were normalized against the enrichment at a control region on chromosome 11 (Gapdh pseudogene). Data represent the mean value of 3 (RNAP II) or 2 (histone modification) independent experiments performed in triplicate.
The Cd19 enhancer is reorganized and accessible before PAX5 expression. (A) DNaseI hypersensitivity assays were performed in bone marrow–derived macrophages (BM-Mø), wild-type (Pax5+/+), and Pax5−/− pro-B cells and Pax5-ER cells induced with OHT for 0, 2, 6, and 24 hours. DNaseI-treated DNA was analyzed by Southern blot as described in Figure 1. (B) RNAP II recruitment, the levels of monomethylated histone H3 lysine 9 (H3K9), monomethylated, and trimethylated histone H3 lysine 4 (H3K4) were measured by ChIP assays in Pax5-ER cells induced with OHT. The enrichment was measured at the promoter, enhancer, and a downstream region at +10 kb. The values were normalized against the enrichment at a control region on chromosome 11 (Gapdh pseudogene). Data represent the mean value of 3 (RNAP II) or 2 (histone modification) independent experiments performed in triplicate.
Cd19 cis-regulatory elements are differentially demethylated in development
The results demonstrate that, even before commitment to the B lineage, Cd19 chromatin was remodeled. To assess whether this was also true for primary cells, we analyzed bone marrow cells at various stages of differentiation as depicted in Figure S3A. The expression of Cd19 mRNA was B cell–specific, and the level of the expression increased with cell differentiation (Figure S3B). The expression of Pax5 mRNA preceded that of Cd19 and increased as the differentiation progressed.
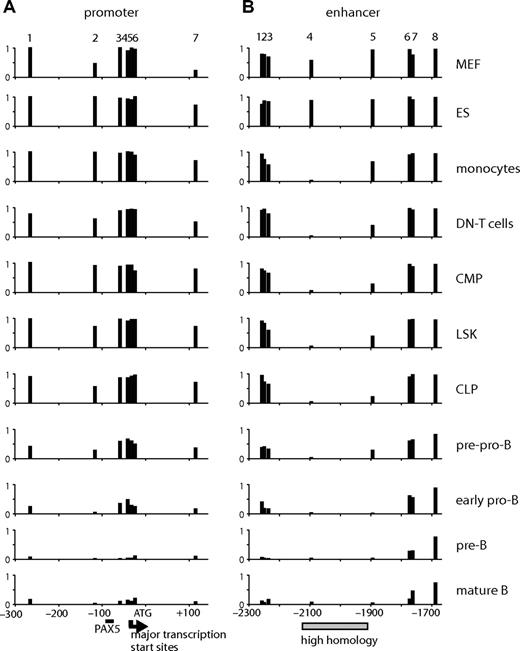
Because only a small number of primary cells (∼104 to 105) could be obtained, it was not feasible to perform DHS mapping or ChIP assays. We have previously shown that the selective demethylation of specific CpG dinucleotides is one of the first steps in the activation of specific cis-regulatory elements.36 We therefore measured the level of the methylation at CpGs using bisulfite sequencing as an indication of the epigenetic state of the gene. To examine multiple samples and to minimize the cloning step bias, we directly sequenced the PCR products of the bisulfite-modified strand and quantified signal intensities at specific CpGs. The DNA sequence of the analyzed regions and examples of sequencing reactions are shown in Figure S4A and S4B, respectively. As seen in Figure 5A, the level of methylation at the promoter was inversely correlated with the level of gene expression. The cytosine residues near the transcription start site and at +112 became coordinately demethylated at the pre-pro-B stage where gene expression was first detected and were further demethylated during B-cell differentiation, probably reflecting the recruitment of active RNAP II to this region. CpGs at the enhancer were demethylated with different kinetics. CpG at −2094 (CpG 4) was completely demethylated in all hematopoietic cells. Removal of methyl-CpG at −1894 (CpG 5) occurred with slower kinetics, but a significant amount of the methyl mark (> 50%) was already removed in progenitors (LSK, lineage−/SCA-1+/c-KIT+; CMP, common myeloid progenitors; CLP, common lymphoid progenitors). The level of methylation at this CpG was further reduced during B-cell differentiation and increased in monocytes. Much slower kinetics of demethylation were observed outside of the enhancer core, suggesting that demethylation initiates at the core and spreads to adjacent regions during B-cell differentiation.
CpG methylation assay at the promoter and enhancer of Cd19 in cells at various differentiation stages. The level of CpG methylation was measured by bisulfite sequencing using genomic DNA derived from MEF, ES cells, monocytes (ER-MP20+ bone marrow cells), CD4−/CD8− (DN) T cells, progenitors (CMP, LSK, CLP), pre-pro-B, early pro-B, pre-B, and mature B cells. The purification profile can be found in Figure S3. The assay was performed on 7 CpGs at the promoter (−300 to + 120) (A) and 8 at the enhancer (−2300 to −1650) (B). The numbers at the top of the panels represent the CpGs found at the promoter (1-7) or the enhancer (1-8) (the exact positions are indicated on the sequence in Figure S4). The numbers at the bottom represent base pairs relative to ATG. The PAX5 binding site is indicated as a horizontal bar. Major transcription start sites ( ) were determined by RT-TDPCR (data not shown). A highly homologous region at the enhancer is indicated by
) were determined by RT-TDPCR (data not shown). A highly homologous region at the enhancer is indicated by  .
.
CpG methylation assay at the promoter and enhancer of Cd19 in cells at various differentiation stages. The level of CpG methylation was measured by bisulfite sequencing using genomic DNA derived from MEF, ES cells, monocytes (ER-MP20+ bone marrow cells), CD4−/CD8− (DN) T cells, progenitors (CMP, LSK, CLP), pre-pro-B, early pro-B, pre-B, and mature B cells. The purification profile can be found in Figure S3. The assay was performed on 7 CpGs at the promoter (−300 to + 120) (A) and 8 at the enhancer (−2300 to −1650) (B). The numbers at the top of the panels represent the CpGs found at the promoter (1-7) or the enhancer (1-8) (the exact positions are indicated on the sequence in Figure S4). The numbers at the bottom represent base pairs relative to ATG. The PAX5 binding site is indicated as a horizontal bar. Major transcription start sites ( ) were determined by RT-TDPCR (data not shown). A highly homologous region at the enhancer is indicated by
) were determined by RT-TDPCR (data not shown). A highly homologous region at the enhancer is indicated by  .
.
Transcription factors, E2A, EBF, and PAX5, sequentially activate the Cd19 enhancer during B-cell development
The state of DNA methylation showed that Cd19 chromatin was already primed in multipotent precursor cells. A number of studies have shown that transcription factors can bind to gene loci in the absence of gene expression.37,38 To test whether this is also true for Cd19, we performed in vivo DMS footprinting. Consistent with the DNA methylation analysis, the enhancer was already accessible to protein binding at the LSK stage, as indicated by a protected guanine at −2036 (Figure 6A, quantification depicted in Figure S5) at the putative E47 binding site (Figure 3B). This was observed in bone marrow-derived progenitors, B lymphocytes, and thymic T cells, but not in MEF. Progressively during B-cell differentiation, an EBF binding site became occupied as indicated by the strong protection of 3 guanines at −2023 to −2021. Both E2A and EBF are expressed in cells where Pax5 is mutated,32 and we confirmed that the same footprints were observed in Pax5−/− pro-B cells (Figure 6B lane 2). A guanine residue at −1990 also became hypo-methylated at the late pro-B cell stage but not in Pax5−/− pro-B cells (Figure 6B). This residue overlaps with a putative PAX5 binding site (Figure 3B), suggesting that the DMS footprint observed at −1990 is the result of PAX5 binding. To confirm the specific binding of these proteins, we performed a ChIP assay using E2A, EBF, and PAX5 antibodies. In agreement with the in vivo DMS footprinting assay, both E2A and EBF bound to the enhancer in Pax5-ER cells in the absence of OHT induction and PAX5 bound only after induction (Figure 6C). Note that the enrichment of the enhancer with E2A and EBF was increased after PAX5 induction, which may be an indication of stabilized protein complexes. These results further confirmed that the Cd19 enhancer is reorganized before lymphoid specification as well as gene expression. They also indicate that the enhancer is regulated by the successive binding of transcription factors, E2A, EBF, and PAX5, which are crucial for B-cell specification, commitment, and maintenance.
Changes in transcription factor assembly at the Cd19 enhancer during B-cell differentiation. In vivo DMS footprinting assays were performed on purified primary cells at various stages of B-cell differentiation (A) and pro-B cell lines (B). DMS-modified and piperidine-cleaved DNA was amplified using LM-PCR with primers specific for the enhancer region. Symbols are as in Figure 2. (C) E2A and EBF bind to the enhancer in pro-B cells in the presence or absence of PAX5. ChIP assays were performed using Pax5-ER cells induced with OHT and antibodies specific for E2A, EBF, and PAX5. The enrichment was measured at the enhancer and a downstream region at +10 kb. The value for relative enrichment was normalized against the enrichment at 45 s rRna promoter. Data are representative of 2 independent experiments performed in triplicate.
Changes in transcription factor assembly at the Cd19 enhancer during B-cell differentiation. In vivo DMS footprinting assays were performed on purified primary cells at various stages of B-cell differentiation (A) and pro-B cell lines (B). DMS-modified and piperidine-cleaved DNA was amplified using LM-PCR with primers specific for the enhancer region. Symbols are as in Figure 2. (C) E2A and EBF bind to the enhancer in pro-B cells in the presence or absence of PAX5. ChIP assays were performed using Pax5-ER cells induced with OHT and antibodies specific for E2A, EBF, and PAX5. The enrichment was measured at the enhancer and a downstream region at +10 kb. The value for relative enrichment was normalized against the enrichment at 45 s rRna promoter. Data are representative of 2 independent experiments performed in triplicate.
Discussion
PAX5 mediates the transcriptional activation of Cd19
Transcription of Cd19 is strictly dependent on PAX5. Here we show that PAX5 binding to the promoter initiates (1) the recruitment of RNAP II, (2) chromatin remodeling and formation of a DHS, (3) the acquisition of active histone marks, and (4) possibly the removal of CpG methylation at the promoter (data not shown). These effects of PAX5 on Cd19 contrast with those at Cd79a, another well-characterized PAX5 target gene. Cd79a expression is not completely abolished by removal of PAX524 ; consequently, the low level of expression is mediated by other transcription factors, such as RUNX1, EBF, and E2A. At the Cd79a promoter DNA methylation, which restricts binding of ETS protein, is removed in CLPs before PAX5 expression in a RUNX1-dependent fashion.12 At the Cd19 promoter, CpGs are methylated, and no Cd19 expression occurs before the action of PAX5.
Chromatin at the Cd19 enhancer is primed in multipotent hematopoietic precursor cells and is reorganized during B-cell differentiation
Our data show that chromatin reorganization at the mouse Cd19 locus during hematopoiesis starts at the −2 kb enhancer. DNA methylation and in vivo DMS footprinting analyses revealed that this reorganization occurs in hematopoietic stem cells or multipotent progenitors (LSK cells) before lineage specification. An earlier study at Igll locus also showed gene activation started at the enhancer without its gene expression.5 However, these experiments were performed in an IL-3–dependent mouse pro-B cell line, Ba/F3, which are thought to be committed B-lineage cells as they express both CD79a and CD79b; therefore, the events before the B-cell commitment were uncharacterized. The earliest stage in epigenetic priming was observed at the Cd79a promoter that was partially demethylated in CLPs but not in HSCs.12 The experiments presented in the current study are, to our knowledge, the first evidence that the chromatin of a B cell–specific gene is primed for expression before lineage-specification.
The activation of Cd19 is mediated by the sequential binding of transcription factors essential for B-cell differentiation
Using in vivo DMS footprinting and ChIP assays, we showed that E2A binds to the Cd19 enhancer in hematopoietic progenitors (Figures 6, S4). Although we have not yet performed the necessary functional assays, E2A is the strongest candidate for a transcription factor responsible for reorganization of the enhancer in these cell types. E2A functions as an E47 homodimer specifically in B cells,39-41 whereas it forms heterodimers with other basic helix-loop-helix proteins in other cell types. It will therefore probably form different complexes at the Cd19 enhancer in T cells and B cells, resulting in distinct functions. After its initial activation, the Cd19 enhancer is successively bound by the B cell–specific transcription factors, EBF and PAX5, identifying a distinct order of events by which Cd19 is transcriptionally activated. Our experiments show that EBF binds to the enhancer before PAX5 (Figure 6B) and that the Cd19 enhancer is involved in appropriate expression (Moreau et al26 and current study). This is consistent with the notion that EBF acts as an upstream regulator of PAX57,42 and is necessary for expression of CD19.43 Although EBF and E2A have been demonstrated to function cooperatively, the order of their contribution to B cell–specific gene expression has not been identified. Our experiments clearly show that E2A binds to the Cd19 enhancer before EBF, and this order of factor assembly is mirrored in accumulating evidence showing that E2A acts upstream of EBF and PAX5 (reviewed in Busslinger44 ). We observed that an unidentified protein bound to a putative ETS binding site adjacent to a PAX5 binding site in B lymphocytes (Figure 3A). On the Cd79a promoter, PAX5 and ETS1 form a ternary complex45 and cooperate with E2A and EBF,13 and the possibility exists that this is also the case here.
Another interesting observation was the recruitment of RNAP II at the enhancer before the promoter. This again confirms that the enhancer is functionally active before the onset of mRNA transcription. In contrast to genes whose expression was detected in HSCs at low levels, the expression of these lymphoid genes has to be activated at the onset of commitment and swiftly reach adequate levels. The storage of RNAP II at the enhancer (Szutorisz et al5 and current study) might be the mechanism that enables this rapid gene activation, although whether RNAP II participating in Cd19 transcription is supplied by the enhancer is unknown. The presence of highly enriched RNAP II and low-level H3K4 trimethylation at the enhancer suggests that this element may generate noncoding transcripts, similar to that observed at a number of other gene loci.46,47 However, how this phenomenon connects to enhancer function remains to be determined.
In conclusion, our data show that the developmental activation of Cd19 starts from the enhancer where 3 transcription factors cooperate in programming chromatin in a tissue-specific fashion. This intricate balance of assembly may be disrupted in certain myeloid leukemias where the CD19 is aberrantly expressed.48,49 Besides providing a well-characterized model for the identification of molecular mechanisms governing B cell–specific gene expression, a thorough understanding of Cd19 regulation may help to provide a molecular handle for the identification of transcription factors and signaling events involved in leukemogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank G. Doody and J. Barton for critically reading the manuscript, L. Straszynski for cell sorting, M. Busslinger for review of the manuscript and providing reagents, including antibodies, cells, and plasmids, and R. Barlow for invaluable assistance.
This work was supported by the Leukaemia Research Fund and by the Kay Kendall Leukaemia Fund. H.T. holds an RCUK Academic Fellowship.
Authorship
Contribution: K.W. performed research; C.B. wrote the paper; and H.T. designed and performed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hiromi Tagoh, Section of Experimental Haematology, Leeds Institute of Molecular Medicine, University of Leeds, St James's University Hospital, Leeds LS9 7TF, United Kingdom; e-mail: h.tagoh@leeds.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal