Abstract
We describe a novel syndrome of severe toxic symptoms during intravascular hemolysis due to impaired hemoglobin scavenging in 2 children with acute myeloid leukemia undergoing CD33-directed therapy with the immunotoxin gemtuzumab ozogamicin (GO). A simultaneous high plasma hemoglobin, haptoglobin, and low bilirubin after septicemia-induced intravascular hemolysis indicated abrogated clearance of haptoglobin-hemoglobin complexes. This was further supported by low levels of plasma soluble CD163 and a concordant low number of CD163-expressing monocytes. We show that CD163 positive monocytes and macrophages from liver, spleen, and bone marrow coexpress CD33, thus suggesting that the GO-induced cellular cytotoxicity of CD33 positive cells eradicates a significant part of the CD163 positive monocytes and macrophages. The risk of severe toxic symptoms from plasma hemoglobin should be considered after CD33-targeted chemotherapy when the disease is complicated by a pathologic intravascular hemolysis. Furthermore, the cases provide further circumstantial evidence of a key role of (CD163-expressing) monocytes/macrophages in plasma hemoglobin clearance in vivo.
Introduction
CD33 is a membrane protein expressed on hematopoietic progenitor cells.1 Leukemic blast cells express the CD33 antigen in more than 90% of patients with acute myeloid leukemia (AML),2 which encouraged development of the CD33-targeted drug gemtuzumab ozogamicin (GO, Mylotarg). GO is a recombinant humanized monoclonal CD33 antibody linked to calicheamicin, a potent cytotoxic agent.3 In 2000, GO was approved by the US Food and Drug Administration for the treatment of patients with CD33 positive AML in first relapse,4 and present experience has shown a potent clinical activity.5 Adverse effects of GO treatment are primarily related to severe myelosuppression (infections, fever, bleedings), and sepsis occurs in 16% of all treated patients.5-7 In addition, hepatic venoocclusive (VOD) and hepatotoxicity have been described.8,9
In this study, we present 2 cases of a novel syndrome of severe toxic symptoms during intravascular hemolysis occurring after treatment with GO, characterized by excessive accumulation of plasma hemoglobin despite high haptoglobin levels. The syndrome is suggested to arise due to a GO-induced defect in monocyte-/macrophage-mediated hemoglobin scavenging.
Methods
Patients
Patient 1.
In December 2003, a 5-month-old boy was diagnosed with AML M4/M5. The patient was treated according to the Nordic Society of Pediatric Hematology and Oncology (NOPHO)–AML 93 protocol.10 Consolidation included matched unrelated donor stem cell transplantation. Relapse was diagnosed 18 months after transplantation, and the patient was treated with the FLAG regimen (fludarabine, cytarabine, G-CSF) without response, and hence with 2 doses of GO (7.5 mg/m2) at a 2-week interval resulting in complete remission. After an episode of staphylococcal sepsis, all blood samples became red/brownish and remained so during a 2- to 3-week period. During this period, the patient had abdominal pain, moderate hypertension, persistent high fever, and malaise despite broad-spectrum antibiotics. The patient recovered but died later from progressive disease.
Patient 2.
In December 2005, an 11-month-old girl was diagnosed with AML M5. The patient was treated according to the NOPHO-AML 2004 protocol (ClinicalTrials.gov identifier NCT00476541). The patient was randomized to receive 2 doses of GO (5 mg/m2) with a 3-week interval. The GO-therapy was without major side effects. The patient experienced a relapse 3 months from completing therapy and was treated with FLAG-DaunoXome, 2 doses of GO (6 mg/m2) with a 2-week interval, and NOPHO-AML 2004 induction-course, but without achieving remission. After a suspected severe infection, all blood samples became red/brownish and remained so for the following 3 weeks, during which period she developed moderate hypertension. The patient became critically ill with confusion and convulsions. She did not recover from the hemolytic episode and died from progressive leukemia 72 days from first GO.
Real-time quantitative reverse transcription polymerase chain reaction assay
Total cellular RNA was extracted using QIAamp RNA Blood Mini (Qiagen, Albertslund, Denmark). Reverse transcriptase was carried out with 1 μL total RNA, as described.11,12 cDNAs were amplified using FastStart DNA MasterPLUS SYBR Green I (Roche Diagnostics, Hvidovre, Denmark) and 5 pmol of each primers. The reactions were performed in a LightCycler System (Roche Diagnostics), as described.11,12
Fluorescence-activated cell sorting analysis
Freshly drawn EDTA-stabilized peripheral whole blood samples were stained with a 3-color combination of allophycocyanin (APC)–conjugated anti-CD14 (Diatec.com, Oslo, Norway), fluorescein isothiocyanate (FITC)-conjugated anti-CD33 (Immunotech, Marseille, France) and R-phycoerythrin (R-PE)-conjugated anti-CD163 (Trillium Diagnostics, Scarborough, ME). Isotype-matched PE- and FITC-conjugated mouse immunoglobulins were used as negative controls. All samples were analyzed using a FACSCalibur flow cytometer and compensated for spectral overlap using FlowJo for Macintosh software version 8.3 (TreeStar, San Carlos, CA) as described.12
Enzyme-linked immunosorbent assay
Soluble CD163 was measured using an in-house enzyme-linked immunosorbent assay, as described.13
Immunohistochemistry and light microscopy
Briefly, double immunohistochemistry for CD33 (1:40 dilution; Immunotech) and CD163 (1:100 dilution; Serotec, Hamar, Norway) was performed on formalin-fixed paraffin-embedded bone marrow, liver and spleen sections. The labeling was visualized with 3,3′-diaminobenzidine tetrahydrochloride (DAB; Ventana Medical Systems, Tucson, AZ) with copper enhancement and Enhanced Alkaline-phosphatase red kit (Ventana) for CD163 and CD33, respectively. Sections were counterstained with hematoxylin. All steps were performed using an automated methodology (Ventana BenchMark XT; Ventana). Images were acquired using a Zeiss Axiophot microscope (Carl Zeiss, Jena, Germany) and processed with Adobe Photoshop CS3 (Adobe Systems, Mountain View, CA).
Double immunofluorescence labeling
Formalin-fixed paraffin-embedded bone marrow, liver and spleen sections were deparaffinized and rehydrated in graded alcohol solutions. Antigen retrieval was achieved by boiling in a microwave. The sections were then incubated with FITC-conjugated anti-CD33 (1:2 dilution; Immunotech). After washing, the sections were incubated with biotin-conjugated anti-CD163 (1:20 dilution; a kind gift from J. Schuitemaker, IQ Products, Groningen, The Netherlands). After a further extensive washing step, sections were incubated with streptavidin-conjugated Alexa Fluor 594 (1:200 dilution; Invitrogen, Carlsbad, CA). All antibody incubations were performed at room temperature for 30 minutes. Sections were mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) with 4′,6′-diamidino-2-phenylindole (DAPI) to identify nuclei. Fluorescence was visualized using a Zeiss Axiovert 200M microscope (Carl Zeiss) with a 100× oil-immersion objective. Representative images were acquired using a AxioCam MRm digital camera (Carl Zeiss). Confocal images were acquired using a Zeiss 510 LSM confocal laser scanning microscope (objective Plan-Apochromat 63×/1.4 NA; Carl Zeiss) equipped with an argon-krypton laser. Image processing was done using NIH ImageJ software (http://rsb.info.nih.gov/ij/, version 1.38w, National Institutes of Health, Bethesda, MD) and Adobe Photoshop CS3 (Adobe Systems).
Results and discussion
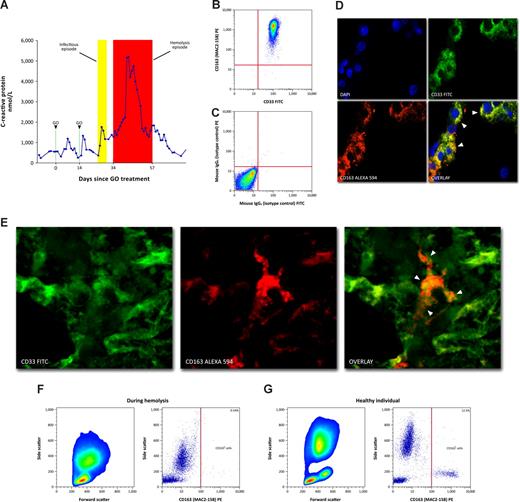
Two patients with AML developed toxic symptoms and extensive red/brownish discoloration of blood samples after infectious episodes weeks after treatment with GO. During the period of discolored blood, the patients appeared septic with persistent high fever, pains, hypertension, and malaise despite broad-spectrum antibiotics. They showed a uniform biochemical pattern of highly elevated plasma hemoglobin without a concomitant increase in bilirubin and depletion of haptoglobin (Table 1). C-reactive protein (CRP; Figure 1A and Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) and lactate dehydrogenase (LDH) were substantially elevated (Table 1).
Patient characteristics and biochemical findings during intravascular hemolysis after treatment with gemtuzumab ozogamicin.
| Case . | Age at GO treatment, y . | Sex . | Diagnose (FAB) . | GO dose, mg/m2 . | Time span from last GO to hemolysis episode, wk . | Assumed cause of intravascular hemolysis . | Free hemoglobin, μmol/L . | Reticulocytes, 109/L . | Lactate dehydrogenase, U/L . | C-reactive protein, nmol/L . | Haptoglobin, μmol/L . | Bilirubin, μmol/L . | Potassium, mmol/L . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt. 1 | 2 | M | AML (M4/M5) | 2 × 7.5 | 3 | Sepsis (Staphylococci) | 151-268 (< 3) | 6-20 (29-83) | 4118-7111 (105-205) | 1283-5212 (< 78) | 34.9-67.8 (2.9-20.0) | 13-22 (<22) | 2.3-5.8 (3.2-4.7) |
| Pt. 2 | 1 | F | AML (M5) | 2 × 5 | 5 | Unknown infection | 67-100 (< 3) | NT (29-83) | 1139-2103 (105-205) | 1333-4505 (< 78) | 26.0-41.4 (2.9-20.0) | 6-19 (<22) | 2.1-4.4 (3.2-4.7) |
| Case . | Age at GO treatment, y . | Sex . | Diagnose (FAB) . | GO dose, mg/m2 . | Time span from last GO to hemolysis episode, wk . | Assumed cause of intravascular hemolysis . | Free hemoglobin, μmol/L . | Reticulocytes, 109/L . | Lactate dehydrogenase, U/L . | C-reactive protein, nmol/L . | Haptoglobin, μmol/L . | Bilirubin, μmol/L . | Potassium, mmol/L . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt. 1 | 2 | M | AML (M4/M5) | 2 × 7.5 | 3 | Sepsis (Staphylococci) | 151-268 (< 3) | 6-20 (29-83) | 4118-7111 (105-205) | 1283-5212 (< 78) | 34.9-67.8 (2.9-20.0) | 13-22 (<22) | 2.3-5.8 (3.2-4.7) |
| Pt. 2 | 1 | F | AML (M5) | 2 × 5 | 5 | Unknown infection | 67-100 (< 3) | NT (29-83) | 1139-2103 (105-205) | 1333-4505 (< 78) | 26.0-41.4 (2.9-20.0) | 6-19 (<22) | 2.1-4.4 (3.2-4.7) |
Results are given as ranges during the periods of hemolysis. The local reference intervals are shown in parentheses.
FAB indicates French-American-British classification21 ; F, female; M, male; and NT, not tested.
CD163/CD33 coexpression and decreased CD163 expression after treatment with gemtuzumab ozogamicin during intravascular hemolysis (patient 1). (A) Time-course of C-reactive protein (CRP) concentrations after GO-treatment in relation to episodes of infection (yellow area) and hemolysis (red area). (B) Coexpression of CD33 and CD163 on CD14 positive monocytes in healthy: Flow cytometric analysis of CD33 and CD163 expression on CD14 positive monocytes in freshly drawn EDTA-stabilized peripheral whole blood from a healthy individual (results are representative of 5 independent experiments). After gating of mononuclear cells (forward- vs side-scatter) and monocytes (CD14+), cells were replotted with CD33 FITC (FL1) versus CD163 (MAC2-158) PE (FL2). (C) Nonspecific FITC- and PE-conjugated IgG isotype-matched controls served as negative controls. (D) Double immunofluorescence microscopy revealed the simultaneous presence of CD33 and CD163 on Kupffer cells (top left panel: DAPI, blue immunofluorscence; top right panel: CD33, green immunofluorscence; bottom left panel: CD163, red immunofluorescence; bottom right panel: overlay, orange/yellow, marked with white arrowheads; original magnification ×100). (E) Immunofluorescence confocal laser scanning microscopy revealed a colocalization of CD33 and CD163 in bone marrow sections from patient 1 (relapse of acute myeloid leukemia before GO-treatment; left panel: FITC-conjugated anti-CD33, green immunofluorscence; middle panel: biotin-conjugated anti-CD163/streptavidin-conjugated Alexa-Fluor 594, red immunofluorescence; right panel: overlay, yellow, marked with white arrowheads; original magnification ×63). (F,G) Flow cytometric analysis of CD163 expression on peripheral blood cells during the hemolysis episode in patient 1 (F), compared with a healthy individual (G). CD163 positive cells were stained with PE (FL2), showing an almost complete depletion of CD163 positive cells (F; right panel). The results shown here from patient 1 are completely consistent with the data for patient 2, which are shown in Figure S1.
CD163/CD33 coexpression and decreased CD163 expression after treatment with gemtuzumab ozogamicin during intravascular hemolysis (patient 1). (A) Time-course of C-reactive protein (CRP) concentrations after GO-treatment in relation to episodes of infection (yellow area) and hemolysis (red area). (B) Coexpression of CD33 and CD163 on CD14 positive monocytes in healthy: Flow cytometric analysis of CD33 and CD163 expression on CD14 positive monocytes in freshly drawn EDTA-stabilized peripheral whole blood from a healthy individual (results are representative of 5 independent experiments). After gating of mononuclear cells (forward- vs side-scatter) and monocytes (CD14+), cells were replotted with CD33 FITC (FL1) versus CD163 (MAC2-158) PE (FL2). (C) Nonspecific FITC- and PE-conjugated IgG isotype-matched controls served as negative controls. (D) Double immunofluorescence microscopy revealed the simultaneous presence of CD33 and CD163 on Kupffer cells (top left panel: DAPI, blue immunofluorscence; top right panel: CD33, green immunofluorscence; bottom left panel: CD163, red immunofluorescence; bottom right panel: overlay, orange/yellow, marked with white arrowheads; original magnification ×100). (E) Immunofluorescence confocal laser scanning microscopy revealed a colocalization of CD33 and CD163 in bone marrow sections from patient 1 (relapse of acute myeloid leukemia before GO-treatment; left panel: FITC-conjugated anti-CD33, green immunofluorscence; middle panel: biotin-conjugated anti-CD163/streptavidin-conjugated Alexa-Fluor 594, red immunofluorescence; right panel: overlay, yellow, marked with white arrowheads; original magnification ×63). (F,G) Flow cytometric analysis of CD163 expression on peripheral blood cells during the hemolysis episode in patient 1 (F), compared with a healthy individual (G). CD163 positive cells were stained with PE (FL2), showing an almost complete depletion of CD163 positive cells (F; right panel). The results shown here from patient 1 are completely consistent with the data for patient 2, which are shown in Figure S1.
Normally, hemoglobin liberated to plasma during intravascular hemolysis is rapidly bound to haptoglobin and instantly removed from circulation. The macrophage specific CD163 protein may play a major role in this process based on haptoglobin-hemoglobin affinity extraction of this protein in a pure form from liver and spleen, and in vitro studies showing its function as a high capacity high-affinity receptor for uptake of haptoglobin-hemoglobin complexes.14 The concentration of haptoglobin in plasma therefore declines, whereas the concentration of bilirubin increases as a result of HO-1–mediated heme metabolism.14,15 This mechanism prevents oxidative and NO-scavenging toxic effects of hemoglobin. These effects have been thoroughly investigated,16 and the known associated clinical symptoms (eg hypertension, gastrointestinal pain, and clot formation) are seen at even lower concentrations (10-50 μM) of plasma hemoglobin than was present in our patients (> 100 μM, Table 1).16 Only in excessive intravascular hemolysis, when the capacity of haptoglobin is exceeded, will the concentration of extracellular hemoglobin rise.17
Combined high haptoglobin and hemoglobin plasma levels are also seen in blood samples undergoing in vitro hemolysis, but this was excluded by careful sampling and the finding of normal potassium levels. The unusual biochemical findings therefore suggested an accumulation of haptoglobin-hemoglobin complexes in plasma.
Because mature monocytes and tissue macrophages express the CD33 antigen,18 the GO-treatment potentially targets hemoglobin-scavenging monocytes/macrophages. Shortly after the second GO treatment, a period of relatively stable hemoglobin levels was observed in patient 1 without an attendant rise in reticulocyte counts (not shown), which aroused suspicion of reduced reticuloendothelial erythrophagocytosis. To assess whether CD33-targeted chemotherapy leads to eradication of CD163 positive cells, we initially set out to investigate the coexpression of CD33 and CD163. Coexpression of the 2 membrane proteins was verified by flow cytometry of peripheral blood monocytes from healthy individuals (Figure 1B,C), double immunohistochemical staining (not shown), and double immunofluorescence microscopy of healthy liver (Figure 1D) and spleen (not shown) samples. A substantial fraction of CD14+ monocytes expressed both CD33 and CD163, and virtually all CD163+ cells stained positive for the CD33 antigen. In the patients, confocal microscopy (Figure 1E and Figure S1B), immunofluorescence microscopy (not shown), and immunohistochemical staining (not shown) also revealed coexpression and colocalization of CD33 and CD163 on bone-marrow macrophages before GO treatment. During the hemolysis episode, an almost complete absence of CD163 positive cells in the circulation was demonstrated by flow cytometry (Figure 1F and Figure S1C) and CD163 mRNA was low or not detectable (not shown). Further support for a severe elimination of CD163+ cells was obtained by low levels of plasma-soluble CD163 at the resolution of the hemolytic episode (1.60 μg/mL), excluding that the findings were due to a complete shedding of CD163. The levels increased to 7.40 μg/mL during recovery (patient 1).
In the 2 presented cases, the symptoms were related to episodes of severe intravascular hemolysis occurring shortly after infectious episodes, however, the triggering factors for hemolysis could not be retrospectively identified. Because intravascular hemolysis normally accounts for a minor but significant fraction of hemoglobin turnover, it is possible that similar but less pronounced findings are present in a larger part of patients treated with GO.
CD163-expressing macrophages in spleen and liver are thought to play a central role in hemoglobin turnover,14 and the shown coexpression of the CD33 and CD163 receptors supports their involvement in the current syndrome. It is, however, not known if the effect of GO is acting directly on the mature cells or on their myeloid progenitors. Moreover, the low density lipoprotein receptor-related protein (LRP), also known as CD91, provides a backup mechanism for the CD163-mediated hemoglobin clearance. Circulating hemoglobin in plasma causes liberation of free heme which instantly binds to hemopexin. The heme-hemopexin complex is recognized by CD91, an endocytic receptor coexpressing with CD163 in the monocyte/macrophage population.11,12,19 The depletion of CD163+ macrophages may therefore also affect this pathway, although partially because CD91 in contrast to CD163 is expressed in a wide subset of cell types including hepatocytes.20
The present results show the usefulness of CD163 for assessment of monocyte-macrophage involvement in disease that adds to the well-established applications in pathology and in diagnosing and monitoring inflammatory and hematologic disorders.15
In conclusion, we describe 2 cases of intravascular hemolysis and severe toxic symptoms from plasma hemoglobin due to impaired hemoglobin scavenging in AML patients undergoing cytotoxic CD33-directed therapy. Furthermore, we have identified a third GO-treated patient (adult) with similar symptoms and biochemical pattern, but a full set of data were not obtainable in this case. Our data suggest that in cases of increased intravascular hemolysis, patients treated with GO are at risk of developing a severe novel syndrome caused by the exposition to the toxic effects of high levels of plasma hemoglobin. A prospective study in a larger cohort of GO-treated patients may define the risk of development of this serious condition in order to initiate appropriate therapy at an earlier stage. Finally, our findings emphasize the central role for (CD163-expressing) monocytes/macrophages in hemoglobin turnover.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kirsten Bank Petersen and Tom Nordfeld for excellent technical assistance, PhD student Rasmus Beedholm-Ebsen for kind help with confocal microscopy, and consultant Bjarne Kuno Møller for providing fluorescence-activated cell sorting analysis facility and expert advice.
This work was supported by grants from the Danish Medical Research Council (22-03-0355, H.J.M.), The Lundbeck Foundation (305/06, H.J.M.), and a PhD research fellowship from the University of Aarhus (M.B.M.).
Authorship
Contribution: M.B.M. performed research (flow cytometry, real-time quantitative reverse transcription polymerase chain reaction, immunofluorescence/confocal microscopy), analyzed data, and wrote the paper; H.H. planned the study, described clinical cases, and critically reviewed the paper; L.F.H., B.L., and O.J.N. described clinical cases; K.B. performed research (immunohistochemistry); S.K.M. analyzed data and wrote the paper; and H.J.M. planned the study, performed research (enzyme-linked immunosorbent assay), analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Holger Jon Møller, Department of Clinical Biochemistry, Århus Sygehus, Aarhus University Hospital, Nørrebrogade 44, DK-8000 Aarhus C, Denmark; e-mail: hjmol@as.aaa.dk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal