Abstract
Patent lymphatic filariasis is characterized by antigen-specific T-cell unresponsiveness with diminished IFN-γ and IL-2 production and defects in dendritic cell (DC) function. Because Toll-like receptors (TLRs) play an important role in pathogen recognition and TLR expression is diminished on B and T cells of filaria-infected individuals, we examined the effect of live microfilariae (mf) on expression and function of TLRs in human DCs. We show that mf-exposed monocyte-derived human DCs (mhDCs) demonstrate marked diminution of TLR3 and TLR4 mRNA expression compared with mf-unexposed mhDCs that translated into loss of function in response to appropriate TLR ligands. Exposure to mf significantly down-regulated production of IFN-α, MIP-1α, IL-12p70, and IL-1α following activation with poly I:C, and of IL-12p40 following activation with poly I:C or LPS. mRNA expression of MyD88, the adaptor molecule involved in TLR4 signaling, was significantly diminished in mhDCs after exposure to mf. Moreover, mf interfered with NF-κB activation (particularly p65 and p50) following stimulation with poly I:C or LPS. These data suggest that mf interfere with mhDC function by altering TLR expression and interfering with both MyD88-dependent signaling and a pathway that ultimately diminishes NF-κB activity. This down-regulated NF-κB activity impairs mhDC-produced cytokines needed for full T-cell activation.
Introduction
Helminth parasites have evolved immune evasion strategies necessary for their continued transmission. This immune evasion is achieved at the expense of both antigen-presenting cells (APCs) and T cells. Filarial parasites have been shown to induce dysfunction in both dendritic cells (DCs) and Langerhans cells, resulting in diminished capacity of these cells to activate CD4+ T cells.1,2 In addition, Toll-like receptor (TLR) expression and function appear to play an important role in filaria-induced immune dysregulation,3,4 as patent filarial infection has been associated with diminished expression of TLR1, TLR2, and TLR4 and diminished responses to TLR2 ligands in both B and T cells.
We have previously shown that monocytes from filaria-infected patients have a diminished capacity to produce IL-12, IL-10, MIP-1α, IL-1α, IL-8, and MIP-1β in response to Staphylococcus aureus Cowan I bacteria (SAC), a ligand that works through TLR2/TLR4.5 This phenomenon extends to other helminth infections, as children with schistosomiasis have also been shown to have diminished responses to TLR ligands compared with those of uninfected children from the same endemic area.6
TLRs are important initiators of innate immune responses through their ability to recognize a variety of microbial products bearing pathogen-associated molecular patterns (PAMPs).7 Although there have been many studies examining TLR signaling in response to intracellular pathogens (including the parasitic protozoa [reviewed in Gazzinelli and Denkers8 ; Yarovinsky and Sher9 ; and Miyake10 ]), many fewer have examined interaction of the multicellular helminth parasites and the TLR system. Indeed, the majority of these have focused on the glycans of schistosomes and TLR2 and the wolbachial endosymbiont of the filariae and TLR2 and TLR4.11-14 The filarial nematode phosphorylcholine-containing secreted product, ES-62, has been shown to affect on IL-12 and TNF-α production by macrophages and DCs through a TLR4 MyD88-dependent pathway.13
TLR-dependent proinflammatory cascades triggered by infections with protozoan parasites and other microbial agents must be tightly regulated to avoid severe pathology or even mortality. Once activated by microbial PAMPs, TLRs transduce signals through 2 pathways involving distinct adaptor proteins containing Toll/IL-1R (TIR) domains.15,16 MyD88 is one of the adaptors used by each of the TLRs except TLR3, which signals mainly through the TIR domain–containing adaptor-inducing IFN-β (TRIF).17 TLR4, the receptor for LPS, is the only TLR that can use either of the 2 adaptors.18 The end result of TLR signaling is activation of NF-κB, resulting in induction of proinflammatory cytokines or interferon regulatory factor (IRF)–dependent induction of type I interferons.
Previously, we have demonstrated that monocyte-derived human DCs (mhDCs) exposed to live microfilariae (mf) of B malayi become less responsive to activation with SAC/IFN-γ to produce IL-12p40 or IL-12p70. In the present study, we have extended these findings to show that live mf of B malayi modulate TLR3 and TLR4 expression in mhDCs, a process that interferes with the MyD88-dependent signaling pathway and causes a failure to activate NF-κB, ultimately resulting in diminished production of both proinflammatory cytokines and type I interferon following TLR3 and TLR4 engagement.
Methods
Preparation of mf
Live B malayi mf were provided by Dr John McCall (University of Georgia, Athens, GA), as described previously.19 Briefly, live mf were collected by peritoneal lavage of infected jirds and separated from peritoneal cells by Ficoll diatrizoate density centrifugation. The mf were then washed repeatedly in RPMI with antibiotics and cultured overnight at 37°C in 5% CO2.
In vitro generation of mhDCs
CD14+ peripheral blood–derived monocytes were isolated from leukopacks from healthy donors by counterflow centrifugal elutriation under IRB-approved protocols from the Department of Transfusion Medicine, Clinical Center at the National Institutes of Health (Bethesda, MD) and informed consent was obtained in accordance with the Declaration of Helsinki. DCs were generated as described previously.1 Live mf were added on day 6 at final concentrations of 50 000 per well (per 1-2 × 106 mhDCs). This number of mf was chosen to reflect in vivo numbers of mf with 50 000 mf per 1 to 2 × 106 mhDCs that is equivalent to that found in individuals with approximately 1000 mf per mL blood (containing 0.02-0.04 DCs). mhDCs were exposed to live mf for 48 hours, then the cells were harvested at day 8 of culture with Versene/EDTA (Biofluids Division, BioSource International, Rockville, MD), washed twice with PBS (without Ca++/Mg++), counted by trypan blue exclusion, and used for functional studies. mhDCs harvested at day 8 were repeatedly shown 98% pure by flow cytometry (FACSCalibur; Becton Dickinson, Sunnyvale, CA).
In vitro activation of mhDCs
On day 8 of culture, mhDCs exposed for 48 hours or unexposed to live mf were harvested and cultured at 106/mL in a 24-well tissue culture plate in media alone or activated with poly I:C (InvivoGen, San Diego, CA) at 25 μg/mL, or ultrapure LPS (InvivoGen) at 5 μg/mL, or flagellin (InvivoGen) at 100 ng/mL. Supernatants were collected 24 to 72 hours after activation. For TLR2 blocking experiments, mhDCs were exposed to live mf for 48 hours in the presence or absence of neutralizing anti-TLR2 antibody (Abcam, Cambridge, MA). Then the cells were harvested and activated with TLR3 or TLR4 ligands for 72 hours, and supernatants were collected. To test the neutralizing effect of anti-TLR2 antibody, mhDCs were activated with the TLR2 ligand HKLM (InvivoGen) for 48 hours in the absence or presence of anti-TLR2 antibody. The supernatant was collected and tested for cytokine production. Anti-TLR2 antibody neutralized the cytokine production by 50% in these cells (data not shown).
Cytokine measurement
All cytokines were detected in culture supernatants using Searchlight proteome arrays (Pierce Biotechnology, Boston, MA). The sensitivity of detection for IFN-α, IL-6, IL-10, and IL-1β is 0.2 pg/mL; for IL-12p40, 1.1 pg/mL; for IL-12p70, IL-8, and IL-1α, 0.4 pg/mL; for IP-10, 1.4 pg/mL; and for MIP-1α, 3.1 pg/mL.
RNA preparation and real-time reverse-transcription–PCR
Total RNA was prepared from 8 to 10 independent donors using the RNAeasy mini kit (Qiagen, Chatsworth, CA). RNA (1 μg) from mhDCs or mf-exposed mhDCs was used to generate cDNA and then assessed by standard multiplex TaqMan assays (Applied Biosystems, Fullerton, CA) on an ABI 7900HT system (Applied Biosystems). Briefly, random hexamers were used to prime RNA samples for reverse transcription (RT) using MultiScribe reverse transcriptase, after which polymerase chain reaction (PCR) products for TLR1-10, MyD88, TRIFF, TAK1, TOLLIP, or IRF3, as well as an endogenous 18s ribosomal RNA control, were assessed in triplicate wells using TaqMan predeveloped assay reagents. The threshold cycle (CT)—defined as the PCR cycle at which a statistically significant increase in reaction concentration is first detected—was calculated for the genes of interest and the 18S control and used to determine relative transcript levels.
Relative transcript levels were determined by the formula: 1/ΔCT, where ΔCT is the difference between the CT of the target gene and that of the corresponding endogenous reference.
Western blot analysis
mhDCs cultured in 6-well plates were exposed to live mf at 50 000 mf/well (8 × 106 monocytes/well as the starting population and approximately 1-2 × 106 DCs/well at the time of mf exposure) for 48 hours. Both nonadherent and adherent cells were collected and lysis buffer (3× SDS and 1× DTT; Cell Signaling, Canton, MA) was added to the cells. Cell lysates were boiled for 5 minutes; 40 μL protein was run in a 1.5-mm 4% to 12% Tris gel and transferred onto PVDF membranes. After blocking using 5% nonfat milk for 1 hour for TLR3 and TLR4 and overnight for tubulin, the membranes were incubated overnight at 4°C with either rabbit anti-TLR3, -TLR4, or -MyD88 (each from Cell Signaling) or with mouse antitubulin (Sigma-Aldrich, St Louis, MO) for 2 hours. After washing, the membranes were incubated with HRP-conjugated anti–rabbit IgG (at 1:10 000; Amersham, Arlington Heights, IL) or anti–mouse IgG (1:10 000; Amersham) at room temperature for 2 hours. For the tubulin control, the membranes were stripped in stripping buffer (62.5 mM Tris·HCl, pH 6.8, 2% SDS, and 100 mM mercaptoethanol) and reblotted with anti–α-tubulin (Sigma-Aldrich) antibody. To demonstrate the specificity of the antibody to TLR3, TLR4, and MyD88, a peptide blocker (Cell Signaling) for each molecule was incubated with the primary antibody for 1 hour prior to use. The proteins were detected by chemiluminescence (Detection System; Cell Signaling). Tubulin was used as an internal control because of low background detection and a molecular weight distinct from the proteins of interest in this study.
Quantifying Western blot analysis
ImageJ (National Institutes of Health [NIH, Bethesda, MD], http://rsb.info.nih.gov/ij/) was used to quantify the intensity of the bands in immunoblots.
Measuring TLR activity
Human embryonic kidney 293 (HEK293) cells were transfected in 96-well plates (104/well) using TransIT (Mirus Bio, Madison, WI) mixed with the mammalian expression plasmid encoding the indicated TLR, a luciferase reporter driven by canonical NF-κB sites (5X NF-κB luciferase; Stratagene, La Jolla, CA) and empty vector to equal 200 ng/well total DNA. After overnight culture, the cells were doubled in number and were treated with TLR ligands for 24 hours. Cells were then treated with live mf, either 25 000 or 2500 per well (2500 per 4 × 104 cells, equivalent to 50 000 mf per 1-2 × 106 mhDCs) for an additional 24 hours. Cell lysates were assayed for luciferase activity using a luciferase reporter assay system (Promega, Madison, WI). Data reflect the luciferase relative light units detected after stimulation for 24 hours.
Preparation of nuclear extracts
mhDCs exposed or unexposed to live mf were activated with poly I:C at 25 μg/mL, or LPS at 5 μg/mL for 3, 24, 48, and 72 hours. The cells were then harvested, and nuclear extracts were prepared using a commercial kit (Active Motif, Carlsbad, CA) according to the manufacturer's protocol. Once made, the amount of protein in the nuclear extract was quantitated using the Bradford protein assay (Sigma-Aldrich) to measure the amount of protein.
Measurement of NF-κB activity
NF-κB activation was measured and quantified by enzyme-linked immunosorbent assay (ELISA) using a TransAm kit (Active Motif).20
Statistical analysis
The nonparametric Wilcoxon signed rank test was used throughout. All statistical analyses were performed with GraphPad Prism 4.0 (GraphPad Software, San Diego, CA).
Results
Live mf of B malayi down-regulate expression of TLR3, TLR4, TLR5, and TLR7 mRNA in mhDCs
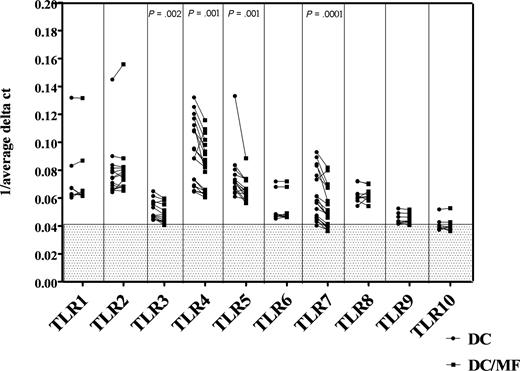
Having shown previously that monocytes from filaria-infected individuals have a diminished cytokine response to SAC (a ligand that works through TLR2/TLR4), we sought to assess whether mf of B malayi had a direct effect on TLR expression. Thus, we generated mhDCs in vitro from elutriated monocytes and exposed the mhDCs to live mf of B malayi for 48 hours and examined TLR expression using real-time quantitative RT PCR. As seen, mhDCs showed basal mRNA expression of human TLR1 to TLR8, a very low expression of TLR9, and almost no expression of TLR10 (Figure 1). Exposure of these mhDCs to live mf, however, resulted in a significant down-regulation of TLR3 (P = .002), TLR4 (P = .001), TLR5 (P = .001), and TLR7 (P < .001) compared with mf-unexposed mhDCs (Figure 1). The decrease in the mRNA expression of TLRs was observed as early as 5 hours (data not shown) and was sustained at 48 hours of exposure of mhDCs to live mf (Figure 1). Moreover, this down-regulation was shown to be specific only to mhDCs, whereas in macrophages derived from the same donor monocytes and exposed to mf, there was no reduction in expression of these TLRs (data not shown).
Live mf of B malayi down-regulate mRNA expression of TLR3, TLR4, TLR5, and TLR7 in mhDCs. mRNA expression in mhDCs and mhDCs exposed to live mf for 48 hours is represented as 1/average ΔCT. The higher the number, the higher the expression of the gene. Results of 7 to 16 independent experiments are shown; each data point and line represent one donor within an experiment. 1/average ΔCT less than 0.041 in the gray box represents no mRNA expression.
Live mf of B malayi down-regulate mRNA expression of TLR3, TLR4, TLR5, and TLR7 in mhDCs. mRNA expression in mhDCs and mhDCs exposed to live mf for 48 hours is represented as 1/average ΔCT. The higher the number, the higher the expression of the gene. Results of 7 to 16 independent experiments are shown; each data point and line represent one donor within an experiment. 1/average ΔCT less than 0.041 in the gray box represents no mRNA expression.
Live mf of B malayi down-regulate TLR4 but not TLR3 protein expression
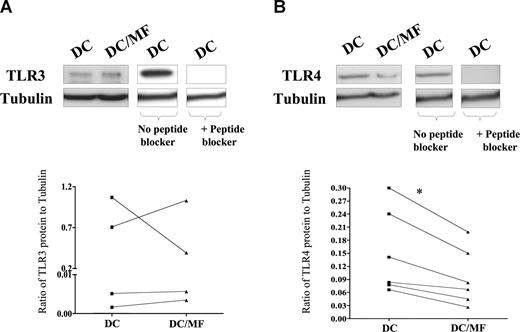
Having demonstrated that live mf diminish mRNA expression of TLR3 and TLR4, we next examined the effect of mf on the protein expression of these 2 TLRs (Figure 2A,B). As seen by immunoblot analysis, our data first demonstrated that mhDCs express measurable TLR3 and TLR4 protein but, more importantly, that following exposure to live mf, TLR4 protein expression (Figure 2B) was markedly down-regulated. Indeed, based on normalization to an internal tubulin control in all donors tested, the ratio of TLR4 to tubulin was diminished in mf-exposed mhDCs compared with unexposed cells (Figure 2B). Furthermore, we were able to detect a 120-kDa TLR3 protein, but we did not observe a consistent change after mf exposure in every donor. Moreover, the 120-kDa and 110-kDa bands seen in mhDCs were specific to TLR3 and TLR4, respectively, as the specific TLR-3 and TLR-4 peptide blockers inhibited binding of anti-TLR3 or anti-TLR4 antibody (Figure 2A,B).
Live mf of B malayi down-regulate TLR4 protein expression. mhDCs were exposed to live mf for 72 hours. TLR3 (A) and TLR4 (B) expression using immunoblot analysis in 1 representative of 4 (for TLR3) and 1 representative of 6 (for TLR4) different donors. Graphs to the side of each blot demonstrate the ratio of TLR to tubulin for each of the 4 or 6 donors. *P = .03.
Live mf of B malayi down-regulate TLR4 protein expression. mhDCs were exposed to live mf for 72 hours. TLR3 (A) and TLR4 (B) expression using immunoblot analysis in 1 representative of 4 (for TLR3) and 1 representative of 6 (for TLR4) different donors. Graphs to the side of each blot demonstrate the ratio of TLR to tubulin for each of the 4 or 6 donors. *P = .03.
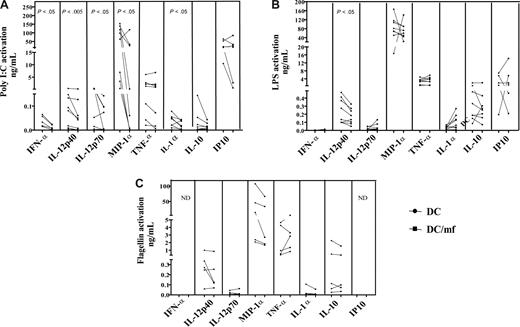
Live mf of B malayi down-regulated production of cytokines in response to TLR3 ligand poly I:C and TLR4 ligand LPS
To determine whether mf-induced mRNA down-regulation of TLR3, TLR4, and TLR5 in mhDCs resulted in diminished responsiveness to the appropriate TLR ligands, we cultured mf-unexposed or -exposed mhDCs either in media alone or in the presence of LPS, poly I:C, or flagellin, a TLR-5 ligand, for 72 hours and examined the production of cytokines known to be produced following TLR stimulation. Of many cytokines tested including IL-18 and IL-8 (data not shown), exposure to live mf significantly down-regulated production of IFN-α, MIP-1α, IL-12p70, and IL-1α following activation with poly I:C (Figure 3A), and of IL-12p40 following activation with poly I:C or LPS (Figure 3A,B). Furthermore, production of TNF-α (7 of 9 donors), IL-10, IL-6 (both, 6 of 9 donors), and IP10 (5 of 7 donors) was down-regulated in mf-exposed mhDCs following activation with poly I:C (Figure 3A). This down-regulation of cytokine was not due to cell death in mhDCs (previously shown to be caused by live mf), as the same number of viable mf-exposed or -unexposed mhDCs were activated with TLR ligands. Moreover, not all cytokines measured are down-regulated following TLR ligand activation. For example, IL-8 and IL-18 expression were unchanged (data not shown). Similarly, gene expression of only TLR3, TLR4, TLR5, and TLR7 is down-regulated (Figure 1; and not TLR1, TLR2, TLR6, or TLR8). In addition, although only 15% to 30% of DCs undergo apoptosis after encountering mf, production of cytokines such as TNF-α, IL-8, and RANTES was up-regulated in DCs (that survive) after mf exposure.1
Live mf of B malayi down-regulate production of mhDC cytokines in response to a TLR3 ligand and to a TLR4 ligand but not to a TLR5 ligand. mhDCs were exposed to live mf for 48 hours and harvested, and viable cells were activated with the TLR3 ligand poly I:C (A), the TLR4 ligand LPS (B), and the TLR5 ligand flagellin (C) for 72 hours. Data presented are net production (spontaneous production subtracted). Each line represents an independent donor. ND indicates not done.
Live mf of B malayi down-regulate production of mhDC cytokines in response to a TLR3 ligand and to a TLR4 ligand but not to a TLR5 ligand. mhDCs were exposed to live mf for 48 hours and harvested, and viable cells were activated with the TLR3 ligand poly I:C (A), the TLR4 ligand LPS (B), and the TLR5 ligand flagellin (C) for 72 hours. Data presented are net production (spontaneous production subtracted). Each line represents an independent donor. ND indicates not done.
Of interest, exposure of mhDCs to mf followed by activation with flagellin did not alter production of any of these cytokines (Figure 3C). Similar results were observed as early as 24 or 48 hours following activation with each ligand (data not shown). Moreover, although TLR7 mRNA was expressed in mhDCs (and also is significantly affected by live mf), this TLR7 has been shown to be nonfunctional in mhDCs, as imiquimod (a TLR7 ligand) failed to induce cytokine production in these cells (data not shown).
Because diminution in both the expression and function of TLR3 and TLR4 (but not of TLR5) appeared to be induced by mf exposure, and as TLR7 is not functional in mhDCs, the focus of the present study concentrated on TLR3 and TLR4.
Live mf of B malayi do not directly trigger TLR3 or TLR4
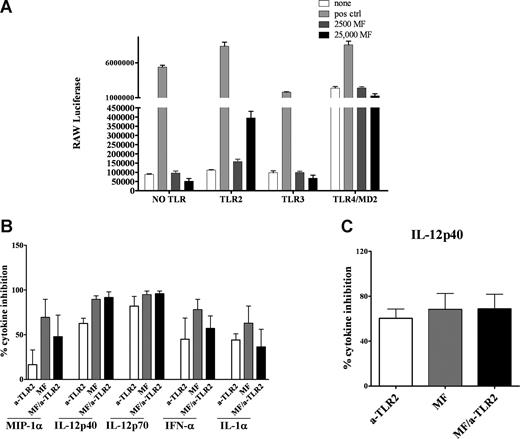
Because live mf down-regulated mRNA expression of TLR3 and TLR4 in mhDCs, and because there was diminished cytokine production in mf-exposed mhDCs in response to either TLR3 or TLR4 ligands, we predicted that mf might directly activate these TLRs leading to down-regulation of TLR expression (a mechanism perhaps to prevent further cell activation). Therefore, we tested the ability of mf to activate TLR3 and TLR4 directly. HEK293 cells were transfected with TLR3, TLR4, or TLR2 (a TLR that was not regulated by mf preexposure), or were used without any TLR transfection (HEK293 cells constitutively express TLR5). The cells were then exposed to the appropriate ligand or live mf at 2 different doses (2500 or 25 000) for 24 hours. As shown in Figure 4A, live mf failed to activate TLR3, TLR4, or TLR5 directly, but activated TLR2, based on luciferase reporter expression in specific TLR transfectants. This suggested that although mRNA levels of TLR3 and TLR4 are modulated by mf exposure, mf do not directly activate them. Instead, mf activate TLR2.
Live mf of B malayi do not trigger TLR3 or TLR4 signaling pathways. (A) HEK293 cells transfected with different TLR (HEK293 cells endogenously express TLR5; no TLR is TLR5) were stimulated for 24 hours with 100 ng/mL flagellin (for “no TLR” to test TLR5), 2 μg/mL poly I:C, 100 ng/mL LPS, 1 μg/mL Pam3Cys or live mf at 2500 or 25 000 per well. Data are expressed as luciferase units. (B,C) Percentage inhibition of cytokines was calculated in 48-hour mf-exposed DCs with or without α-TLR2 antibody and compared with unexposed DCs. All cultures were then activated with either (B) poly I:C or (C) LPS for 72 hours. Cytokines shown (IFN-α, IL-12p40, IL-12p70, MIP-1α, and IL-1α for poly I:C activation and IL-12p40 for LPS activation) are the cytokines significantly down-regulated in mf-exposed DCs (Figure 2A,B). Data shown as bar graphs of 3 to 4 independent experiments with means and standard error of the mean.
Live mf of B malayi do not trigger TLR3 or TLR4 signaling pathways. (A) HEK293 cells transfected with different TLR (HEK293 cells endogenously express TLR5; no TLR is TLR5) were stimulated for 24 hours with 100 ng/mL flagellin (for “no TLR” to test TLR5), 2 μg/mL poly I:C, 100 ng/mL LPS, 1 μg/mL Pam3Cys or live mf at 2500 or 25 000 per well. Data are expressed as luciferase units. (B,C) Percentage inhibition of cytokines was calculated in 48-hour mf-exposed DCs with or without α-TLR2 antibody and compared with unexposed DCs. All cultures were then activated with either (B) poly I:C or (C) LPS for 72 hours. Cytokines shown (IFN-α, IL-12p40, IL-12p70, MIP-1α, and IL-1α for poly I:C activation and IL-12p40 for LPS activation) are the cytokines significantly down-regulated in mf-exposed DCs (Figure 2A,B). Data shown as bar graphs of 3 to 4 independent experiments with means and standard error of the mean.
To address whether mf activation of TLR2 was responsible for the effects on TLR3 and TLR4, we used neutralizing antibodies to TLR2 prior to activating mf-exposed mhDCs with LPS or poly I:C. Blocking TLR2 in mhDCs exposed to live mf did not reverse the diminished cytokine production following TLR3 (Figure 4B) and TLR4 (Figure 4C) activation. These data suggest that the effect of mf on TLR3 and TLR4 down-regulation is through pathways other than TLR2.
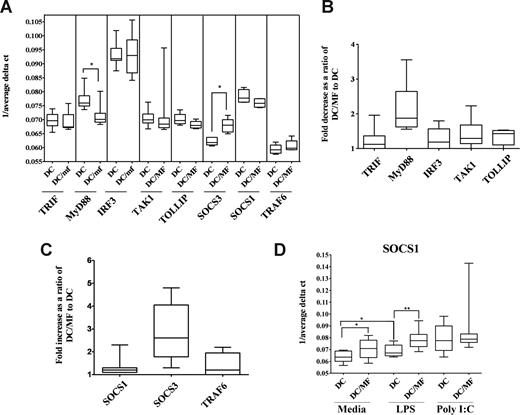
Preexposure to live mf diminishes both mRNA and protein expression of MyD88 but not TRIF and induces mRNA expression of SOCS1 and SOCS3
The diminished TLR response that we observed in mhDCs may not be due solely to mf-induced down-regulation in TLR3 and TLR4 mRNA or protein expression. In fact, the subcellular localization of TLRs or other adapter molecules can have a dramatic impact on response to TLR ligands. It has been demonstrated that upon engagement with their ligands, TLRs recruit specific adaptor molecules that propagate downstream signaling.21 Whereas stimulation through TLR4 activates both a common MyD88-dependent pathway and a MyD88-independent TRIF-dependent pathway, TLR3 stimulation occurs primarily through a TRIF-dependent pathway. Because preexposure to live mf diminished production of IFN-α following poly I:C activation and IL-12p40 following LPS activation, we examined whether these adaptor molecules were responsible for the impairment seen (Figure 5). Our results suggest that 48-hour exposure to mf resulted in a significant decrease in mRNA expression of MyD88 (P = .01) but not TRIF in mhDCs (Figure 5A,B). Furthermore, 48- to 96-hour preexposure to mf led to a slight decrease in protein expression of MyD88 but not TRIF (data not shown). In addition, when we studied the effect of live mf on IRF3, TAK1, TRAF6, or TOLLIP—each involved in activation or regulation of TLR signaling—we were unable to see any change in expression after exposure of mhDCs to live mf (Figure 5A,B) or following activation with either poly I:C or LPS (data not shown).
Preexposure to live mf diminishes mRNA expression of MyD88 in mhDCs while it enhances mRNA expression of SOCS3. (A-C) Results shown as (A) 1/average ΔCT in mhDCs and 48-hour mf-exposed DCs, (B) fold decrease, and (C) fold increase in mf-exposed mhDCs compared with unexposed, and (D) 1/average ΔCT in mhDCs, mf-exposed mhDCs, 72-hour LPS-activated mhDCs, and 72-hour LPS-activated mf-exposed mhDCs using box-and-whisker plots with median indicated by the line, 75th and 25th percentiles indicated by the box, and the range (minimum to maximum) of 6 to 8 independent donors. P values were calculated based on 1/average ΔCT comparing mhDCs and mf-exposed mhDCs. *P < .05; **P < .005.
Preexposure to live mf diminishes mRNA expression of MyD88 in mhDCs while it enhances mRNA expression of SOCS3. (A-C) Results shown as (A) 1/average ΔCT in mhDCs and 48-hour mf-exposed DCs, (B) fold decrease, and (C) fold increase in mf-exposed mhDCs compared with unexposed, and (D) 1/average ΔCT in mhDCs, mf-exposed mhDCs, 72-hour LPS-activated mhDCs, and 72-hour LPS-activated mf-exposed mhDCs using box-and-whisker plots with median indicated by the line, 75th and 25th percentiles indicated by the box, and the range (minimum to maximum) of 6 to 8 independent donors. P values were calculated based on 1/average ΔCT comparing mhDCs and mf-exposed mhDCs. *P < .05; **P < .005.
Furthermore, as it has been reported that SOCS proteins also can inhibit LPS signaling through TLR4,22,23 we measured SOCS1 and SOCS3 mRNA levels in mhDCs after exposure to live mf. Whereas mRNA expression of SOCS1 did not change in mhDCs, preexposure to live mf for 48 hours significantly up-regulated mRNA expression of SOCS3 in these cells (Figure 5A,C). Of interest, 72-hour activation with LPS resulted in significant up-regulation of SOCS1, which was further enhanced when mhDCs were exposed to mf for 48 hours prior to activation (Figure 5D).
Down-regulation of MyD88, TLR3, or TLR4, as well as up-regulation of SOCS3, was seen after 48-hour exposure to mf and prior to activation with either LPS or poly I:C (Figures 1 and Figure 5A-C, and data not shown). Whereas 48-hour mf exposure did not change the mRNA levels of SOCS1 in mhDCs, the enhancement in the level of SOCS1 mRNA was more profound when the cells were first exposed to mf for 48 hours and then cultured either in media alone or activated with LPS (Figure 5D).
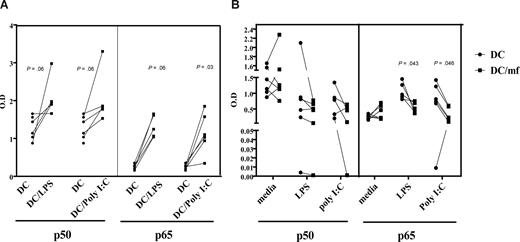
Preexposure to live mf diminishes binding of NF-κB p50 and p65 following poly I:C or LPS activation
Because both MyD88- and TRIF-dependent signaling cascades can lead to activation of NF-κB and production of proinflammatory cytokines, and because TRIF mediates activation of IRF transcription that can result in type I interferon production, we examined whether mf-dependent impairment of mhDC function extends to down-regulation of NF-κB activity. First, stimulation of mhDCs with LPS or poly I:C resulted in activation of both p50 and p65 subunits of NF-κB, which could be detected as early as 30 minutes (data not shown) but become increasingly abundant after 3 hours (Figure 6A). Second, by exposing mhDCs to live mf for 48 hours and then activating them with either poly I:C or LPS, we were able to demonstrate that mf preexposure of mhDCs resulted in diminished binding of both the p50 and p65 subunits of NF-κB as early as 3 hours (Figure 6B). Our results indicate a range of 20% to 70% inhibition of binding of p65 subunit of NF-κB following activation with poly I:C (P = .03), and 4 of 5 donors showed a range of 12% to almost 90% inhibition following activation with LPS in mf-exposed mhDCs compared with the unexposed cells (Figure 6B). The decrease in binding ability of p50 was less profound.
Preexposure to live mf diminishes the NF-κB binding capacity of p50 and p65 following poly I:C and LPS activation. (A) Unexposed mhDCs activated with either LPS or poly I:C for 3 hours. (B) mhDCs were exposed to live mf for 48 hours and then activated with either media alone, poly I:C, or LPS for an additional 3 hours. Nuclear extracts were prepared, and NF-κB activation was measured using an ELISA with OD as the readout. Each line represents an independent experiment.
Preexposure to live mf diminishes the NF-κB binding capacity of p50 and p65 following poly I:C and LPS activation. (A) Unexposed mhDCs activated with either LPS or poly I:C for 3 hours. (B) mhDCs were exposed to live mf for 48 hours and then activated with either media alone, poly I:C, or LPS for an additional 3 hours. Nuclear extracts were prepared, and NF-κB activation was measured using an ELISA with OD as the readout. Each line represents an independent experiment.
Discussion
One hallmark of filarial (and other systemic helminth) infections is their chronicity, felt to reflect the parasites' successful evasion of the host response. Among the many mechanisms proposed to play a role in modulating the host response to benefit parasite survival are regulatory T cells and cytokines,24 conditioned Th2 cells,25 alternatively activated macrophages and iNOS,26 and altered function of APCs, the latter concept given credence by a number of in vitro1,2 and in vivo5 studies. Together, these data suggest that filaria-conditioned APCs are poor inducers of T-cell responses.
The TLR family is the best-characterized class of pattern recognition receptors in humans. Activation of APCs by pathogenic organisms occurs primarily through TLR recognition and signaling. Although bacterial (and nonbacterial intracellular) pathogens typically cause increases in TLR expression,27 down-regulation of TLR expression appears to be an important evasion strategy used successfully by some bacterial pathogens. TLR4 down-regulation and tolerance by LPS and TLR2 down-regulation by bacterial lipoprotein have been implicated commonly as a mechanism of bacteria-induced immune suppression.28,29 Similarly, parasites such as Entamoeba histolytica and Trypanosoma spp have been shown to inhibit immune responses by down-regulating TLR2 expression30 and TLR-mediated signaling.31,32 In previous studies, monocytes from filaria-infected humans also showed inhibited TLR expression and responses to TLR ligation.3,4
Using live mf, the stage of the parasite most likely to interact directly with both the host adaptive and innate immune systems, the present study examined the interaction between the parasite and TLR expression on APCs. As shown in Figure 4, mf did not trigger TLR3 or TLR4 directly, but the parasites' major influence was to inhibit TLR3, TLR4, TLR5, and TLR7 mRNA expression and protein expression (at least for TLR4). In addition, this phenomenon was not due to a generalized suppression of TLR by mf, as the expression of other TLRs such as TLR1 and TLR2 (among others) was unaffected by mf exposure. Furthermore, this was not a failure to detect TLR expression (Figure 1). Most important, this down-regulation of TLR expression translated into diminished responsiveness of mf-exposed mhDCs to TLR3 and TLR4 ligation.
Notably, this down-regulation was specific to DCs, as the TLRs from macrophages of the same donor were not affected by live mf (data not shown). Whether the differences between macrophages and DCs reflect only intrinsic differences between the cell types or differences in tissue distribution (macrophages being commonly resident in tissues, whereas DCs are more mobile) awaits clarification, although microarray analysis suggests that these 2 cell types share similar baseline gene expression patterns.33
As the diminished responsiveness was specific to TLR3 and TLR4 (not TLR5, and TLR7 is not functional in mhDCs), we chose to focus on the downstream events of mf/TLR interaction of these 2 TLRs. One novel aspect of our current study, however, was the ability to detect the TLR3 protein by immunoblotting. Although our data strongly demonstrate that mhDCs express the 120-kDa TLR3 protein, the fact that live mf fail to alter (qualitatively, at least) its expression may point to transcriptional regulation, altered kinetics of expression, or degradation of the TLR3 protein. Obviously, TLR3-independent activation by poly I:C cannot be excluded, as poly I:C–induced cellular activation has been shown to occur in mice deficient in TLR3.34 Furthermore, it is likely that down-regulation of protein expression does not account for the lack of TLR response. Cellular localization of TLRs is critical for ligand recognition; for example, TLR4 is expressed at the cell surface, and intracellular retention prevents responses to LPS.35 Localization is especially important for TLRs that recognize nucleic acids, as they are not expressed at the cell surface and require appropriate intracellular trafficking to recognize DNA and RNA.36 For example, TLR3 is expressed intracellularly, likely in vessicles prior to ligand exposure (C.A.L., personal observation, May 11, 2004). Since the response to TLR3 ligands is sensitive to endosomal acidification inhibitors, localization to vessicles is critical for a response. If TLR3 is prevented from reaching vessicles, or if it is actively diverted from endosomes, exposure to ligands would fail to elicit a response. Therefore, although protein levels for TLR3 are not modulated, mf exposure could alter the localization of TLR3. Thus, the lack of response to TLR ligands in mhDCs may be due to regulation not at the mRNA or protein levels but at the level of subcellular localization.
It has been shown by several investigators that MyD88 is essential for resistance to parasitic or bacterial infection37-39 ; however, in our hands, although mf had no effect on expression of TRIF, it significantly down-regulated the mRNA expression of MyD88 in mhDCs (Figure 5A,B), suggesting that mf have an effect on the regulation of this adaptor molecule. This down-regulation was observed following exposure to mf and prior to TLR4 or TLR3 ligand activation (Figure 5 and data not shown). Indeed, although there are no data implicating a MyD88-dependent pathway following TLR3 activation, we have shown that activation with poly I:C (but not LPS) up-regulates mRNA expression of MyD88 mRNA (data not shown). This finding is similar to microarray data40 demonstrating up-regulation of MyD88 in human peripheral blood mononuclear cells following poly I:C activation. This up-regulation of MyD88 may also be an indirect effect of poly I:C–dependent production of cytokines such as type I interferons.
In addition, live mf up-regulated mRNA expression of SOCS3 in mhDCs prior to any TLR ligand activation; following LPS activation, live mf enhanced expression of SOCS1 (Figure 5A-C). Of interest, the SOCS family of proteins has been suggested to be involved in inhibition of LPS signaling through TLR4 and might be responsible for LPS tolerance.22,23 SOCS1 also negatively regulates TLR2 and TLR4, possibly through a decrease in type I IFN autocrine/paracrine signaling following TLR-mediated type I interferon secretion.41,42 Whether in our system the up-regulation of SOCS1 (following LPS activation) and SOCS3 (prior to LPS activation) by live mf is a direct or indirect effect of SOCS-mediated inhibition of TLR signaling remains to be elucidated. Another molecule known to negatively regulate TLR signaling is TOLLIP. It has been shown that TOLLIP can block activation of NF-κB through TLR2 or TLR443,44 ; however, in the present study, 48-hour exposure to live mf did not alter gene expression of TOLLIP in mhDCs.
We also examined late (downstream) signaling events by measuring the effect of mf on NF-κB activation. We were able to show that mhDC exposure to live mf followed by activation with either poly I:C or LPS results in diminished binding activity of the p65 and to a lesser degree p50 subunits of NF-κB. These data could help explain how both IFN-α and IL-12p40 are modulated in mf-exposed mhDCs following activation of TLR ligands. Activation of NF-κB has also been shown to be inhibited by other parasites such as Toxoplasma gondii and Leishmania mexicana. Infection with T gondii induces rapid IκB phosphorylation and degradation but results in the failure of NF-κB translocation to the nucleus.45,46 This failure also occurs when infected macrophages are stimulated with LPS.47
Our data collectively suggest that live mf of B malayi negatively regulate expression of TLR3 and TLR4, down-regulate expression of the adaptor molecule MyD88, and diminish the binding capacity of p50 and p65 subunits of NF-κB. These effects may translate directly to diminished cytokine production by mhDCs in response to TLR3 and TLR4 ligands and, as a result, in loss of function. How these extracellular parasites exert their effect in mhDCs is not fully understood. We have previously shown that the effect of live mf on mhDCs may be through both a contact-dependent interaction between the parasites and the cells and by their secretion of soluble factors.1 Differential regulation of TLRs may be the indirect effect of live mf. Although our data suggest that live mf do not activate TLR3 and TLR4 directly, the down-regulatory effect of mf can be through up-regulation of other cytokine or soluble factors involved in regulation of TLRs. We know that live mf up-regulate expression of SOCS1 and SOCS3, and we are in the process of investigating the relationship between SOCS up-regulation and TLR down-regulation. In addition, the possibility that live mf may bind particular TLRs but not signal cannot be formally excluded. This concept has been given credence in murine studies by Goodridge et al,13 in which ES-62 of the filarial nematode, Acanthocheilonema vitiae, induces low production of IL-12 and TNF-α in a TLR4- and MyD88-dependent manner but leads to subsequent inhibition of cytokines such as IL-12, TNF-α, and IL-6 by the TLR4 ligand LPS. Another possibility is that direct binding of mf to TLR2 could have a negative regulatory affect on TLR3 and TLR4 function. Our data suggest that although mf can activate TLR2 directly (Figure 4A), this activation does not negatively regulate TLR3 and TLR4 function, as blocking the TLR2 pathway using a neutralizing anti-TLR2 antibody did not reverse the cytokines down-regulated in mf-exposed mhDCs following activation with either TLR3 or TLR4 ligands (Figure 4B,C).
We know that professional APCs such as mhDCs, Langerhans cells, and macrophages are capable of internalizing antigen from filarial worms (Semnani et al2 and data not shown) and that monocytes of filaria-infected individuals are studded with filarial antigen.5 Whether ligand/receptors expressed by these worms are involved in the process of altering APC function and/or filarial parasites' secreted products are directly altering the function of these cells is one aspect of our future work.
What the filarial parasite gains by modulating host TLR expression remains an open question. Our data indicate that live mf down-regulate the expression of these TLRs and the expression of downstream signaling molecule MyD88. This down-regulation in the gene expression results in a decrease in the function of these cells and their response to TLR ligands. This, in turn, may translate in clinical settings to a decreased response of microfilaremic patients to secondary infection with bacteria or viruses. Compromised TLR expression and function on APCs could certainly explain the diminished immune responses observed in bystander antigen and routine vaccinations48,49 seen in helminth-infected patients. Thus, these data provide insight into the central role TLR dysregulation plays in APC function and suggests strategies for overcoming this dysregulation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Manju Joshi and Joseph Kubofcik for their technical help; Drs Julie Tierney and Matthew Fry from Cell Signaling for technical help and advice on TLR immunoblots; Drs Darrell Hurt and Mariam Quinones in the Bioinformatics and Computational Biosciences Branch of National Institute of Allergy and Infectious Diseases (NIAID, Bethesda, MD) for help with graphics; and Drs Alan Sher, Felix Yarovinsky, Siddhartha Mahanty, and Brian Kelsall for critical reading of the paper, discussions, and useful advice. We also thank NIAID intramural editors Brenda Rae Marshall and Nancy Shulman for assistance.
Because R.T.S., P.G.V., and T.B.N. are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. You can establish rights outside of the United States subject to a government use license.
This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: R.T.S. participated in all aspects of the study including the design, research, data analysis, and writing of the paper; P.G.V. and C.A.L. participated in the research, data analysis, and writing the paper; S.M and H.S participated in research; T.B.N. participated in design, analysis, and writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roshanak Tolouei Semnani, LPD, NIAID, 4 Center Dr, National Institutes of Health, Bethesda, MD 20892; e-mail: rsemnani@niaid.nih.gov.