Abstract
The regulation of CD4 T-cell numbers during an immune response should take account of the amount of antigen (Ag), the initial frequency of Ag-specific T cells, the mix of naive versus experienced cells, and (ideally) the diversity of the repertoire. Here we describe a novel mechanism of T-cell regulation that potentially deals with all of these parameters. We found that CD4 T cells establish a negative feedback loop by capturing their cognate major histocompatibility class (MHC)/peptide complexes from Ag-presenting cells and presenting them to Ag-experienced CD4 T cells, thereby inhibiting their recruitment into the response while allowing recruitment of naive T cells. The inhibition is Ag specific, begins at day 2 (long before Ag disappearance), and cannot be overcome by providing new Ag-loaded dendritic cells. In this way, CD4 T-cell proliferation is regulated in a functional relationship to the amount of Ag, while allowing naive T cells to generate repertoire variety.
Introduction
Because the frequency of T cells specific for any antigen (Ag) is low, T-cell proliferation is an important part of primary immune responses. However, proliferation must stop at some point to allow Ag-specific T cells to become effectors (and to accommodate a limited body size). In a typical primary immune response, CD4 T cells proliferate extensively and generate effector cells before a contraction phase sets in. The extent of proliferation depends upon the initial frequency of the Ag-specific T cells and is therefore much greater for naive T cells than for the more numerous memory T cells.1-3 Because memory and naive cells reach the same plateau, even though memory cells respond faster, there must be mechanisms that regulate T-cell proliferation early during an immune response.4,5 It has been suggested that T-cell proliferation is related to the disappearance of Ag or antigen-presenting cells (APCs),6-8 to exhaustion of the APCs,9 to suppression by regulatory T cells,10 or to competition among responding T cells.11-14 However, disappearance or exhaustion of APCs should generate higher final plateaus for the faster-responding memory T cells; and pure competition for waning Ag would rapidly lead to preferential expansion of high-avidity T-cell clones, and thus to a narrowing of the repertoire. This is not observed for CD4 T-cell responses, where the avidity range remains quite wide.15
While studying regulation of a localized CD4 immune response, we found yet another mechanism. Responding CD4 T cells capture and present their cognate MHC/Ag complexes in a manner that is strongly inhibitory for activated/memory CD4 T cells but not for naive T cells. This inhibition regulates the intensity of the immune response in relation to the amount of presented Ag while keeping the repertoire diverse, as new naive T cells can still enter the immune response.
Methods
Mice
Marilyn TCR-transgenic Rag2−/− mice are specific for the Dby-H-Y male antigen presented by Ab.16 For Marilyn T cells expressing diphtheria toxin receptor (DTR), we crossed Marilyn Rag2−/− CD45.1 mice to Lat-DTR knockin mice (harboring, in the 3′ untranslated region of the Lat gene,17 a human diphtheria toxin receptor (DTR) cassette, driven by an internal ribosomal entry site [A.K. and B.M., manuscript in preparation]) to finally obtain Marilyn-Lat-DTR.Rag2−/−.CD45.1/2 mice. B6-OT-II18 and B6 mice were from CRL (Charles River Laboratories, L'Arbresle, France). Live animal experiments were done according to French Veterinary Department guidelines.
Cell preparation
Effector and memory T cells were generated in vivo as described,19 by transferring 106 Marilyn or OT-II LN cells into CD3ϵ−/− hosts together with 3 × 106 mitomycin-treated male splenocytes or LPS-matured bone marrow dendritic cells (DCs) loaded with OVA323-339 peptide (Neosystem, Strasbourg, France), respectively. Effector or memory T cells were purified from the spleen and lymph nodes 5 days later or at least 6 weeks later, respectively, using anti–CD45.1-PE antibody and anti-PE microbeads. Such positive purification does not alter T-cell behavior (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Naive, effector, or memory T cells were labeled with 5 μM CFSE (Invitrogen, Carlsbad, CA) in PBS containing 0.1% BSA, for 8 minutes at 37°C.
Depletion experiment and flow cytometry
To deplete Marilyn-Lat-DTR-Rag2−/−T cells in vivo, we injected 500 ng diphtheria toxin (DT; List Biological Laboratories, Campbell, CA) intravenously twice, 8 hours apart, the day before the second transfer.
Four- to 8-color cytometry was performed with directly conjugated antibodies: anti–CD45.1-phycoerythrin (PE), anti–Vβ6-PE, anti–Vα2-PE, anti–BrdU-PE, anti–CD62-L-PE, anti–CD44-PE, anti–IAβ-PE, anti–CD45RB-PE, anti–βTCR-cychrome, anti–CD45.2-peridinin chlorophyll protein (PerCP)—cyanin 5.5 (Cy5.5), anti–CD69-biotin, anti–CD44-biotin, anti–Vα2-biotin, and anti–Vβ6-biotin; biotinylated antibodies were revealed with streptavidin-allophycocyanin APC/APC-Cy7 (APC or APC-Cy7; Pharmingen, San Diego, CA), and analyzed using FacsCalibur, Aria, or LSRII flow cytometers (Becton Dickinson, San Jose, CA).
Generation of dendritic cells and immunizations
Bone marrow–derived dendritic cells (BMDCs) were generated by 10- to 15-day culture in granulocyte-macrophage colony-stimulating factor (GM-CSF)–containing conditioned medium as previously described,20 matured by 20-hour treatment with 10 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich, St Louis, MO), pulsed with 40 nM Dby (NAGFNSNRANSSRSS) peptide for 2 hours, and washed 3 times before injection. This peptide dose is just at plateau for in vitro proliferation and at 30% of maximum for IFN-γ production (Figure S2G and H, respectively). In some experiments (Figure 4A), BM was obtained from mice expressing a fusion protein of Ab-GFP.21
DCs were injected into the footpads of both hind feet of female B6 mice that had been injected intravenously the day before with 106 LN cells from CD45 congenic Marilyn mice.
In vitro experiments
CFSE-labeled Dby-loaded BMDCs (2 × 106) and CD45.2 Marilyn T cells (2 × 106) were cocultured overnight in RPMI-1640 plus 10% fetal calf serum in 6-well plates, negatively sorted (CD11c−/CFSE−) by fluorescence-activated cell sorting (FACS), and fixed with PBS plus 0.01% glutaraldehyde on ice for 1 minute (stopped with 0.2 M glycine-PBS). Fixed Marilyn T cells (5 × 105) were then incubated for 1 or 3 days with 2 × 104 CD45.1 CFSE-labeled naive or memory Marilyn cells that had been purified by FACS-based negative selection (CD11c− for the naive cells from which DCs had been removed by FACS-based negative selection; CD11c− for the naive cells; and CD11c−/CD45.2− for the memory cells). DC contamination was less than 0.02%.
Results
Naive and memory CD4 T cells are differentially recruited into ongoing immune responses in vivo
To unravel the mechanisms controlling CD4 T-cell expansion during a localized immune response, where new and returning T cells continuously enter the draining lymph node (LN), we tracked the responses of a monoclonal population of H-Y/Ab–specific CD4 T cells (from the TCR transgenic [Tg] Rag2−/− Marilyn mouse16 ) that were transferred into normal female B6 mice 1 day before immunizing the recipients in the footpad with LPS-activated female bone marrow–derived dendritic cells (DCs) loaded with the H-Y peptide. To track cell divisions, we labeled the transferred Marilyn T cells with CFSE, and to discriminate them from host cells, we used CD45.1 Marilyn cells and CD45.2 hosts (Figure 1A). For naive cells, we used LN cells from Marilyn mice. For Ag-experienced T cells, we transferred naive Marilyn T cells together with H-Y–expressing male cells into CD3ϵ−/− hosts and recovered them after 5 days (effector cells) or more than 6 weeks (memory cells) later.16
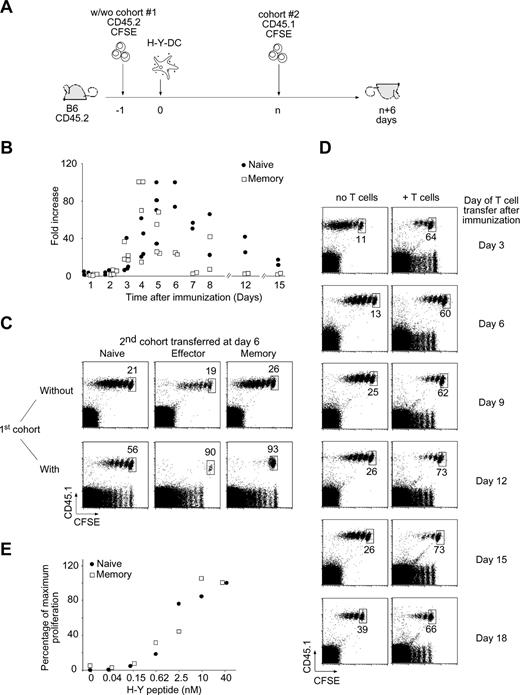
Ag-experienced T-cell proliferation is preferentially inhibited during on ongoing immune response. (A) Two-cohort experimental design. A first cohort of 106 naive CD45.2 CFSE-labeled Marilyn cells was injected intravenously into CD45.2 B6 mice that were immunized by injection of 106 H-Y peptide–loaded LPS-matured DCs into the footpad the next day. After a variable (n) number of days, a second cohort of either 106 naive, 5 × 106 effector, or 2 × 106 memory CD45.1 CFSE-labeled Marilyn cells was injected. CFSE profiles of gated TCR+CD45.1+ cells from the popliteal and inguinal lymph nodes draining the immunization site (DLN) were assessed 6 days after transfer of the second cohort. (B) Kinetics of the response of transferred Marilyn TCR Tg T cells. Naive (106) or memory (2 × 106) CD45.1 CFSE-labeled Marilyn cells were injected intravenously into B6 mice that were primed the next day by injection of 106 H-Y peptide–loaded LPS-matured DCs into the footpad. The percentage of transferred T cells (CD45.1+TCR+) in the draining lymph node was measured at the indicated time points and compared with the percentage measured at day 1 in the absence of antigen. Each dot represents one mouse. Pooled data from 2 independent experiments. (C) Stronger inhibition of Ag-experienced versus naive T-cell proliferation. Naive (left panels), effector (middle panels), or memory (right panels) Marilyn T cells were injected into primed B6 hosts in the absence (top panels) or the presence (bottom panels) of a previous cohort of responding T cells injected 6 days earlier, as in panel A. Dot plots of gated TCR+CD45.1+ cells from the DLN are representative of at least 3 experiments with 2 mice each per group. The percentage of undivided cells, among total Tg Marilyn T cells, is indicated. (D) Antigen persists in vivo at least 18 days. H-Y peptide–loaded LPS-matured DCs were injected into the footpad of female B6 mice, previously injected (right panels) or not (left panels) with naive CD45.2 CFSE-labeled Marilyn LN cells. At the indicated time points, naive CD45.1 CFSE-labeled Marilyn LN cells were transferred intravenously. Dot plots of gated TCR+CD45.1+ cells from the DLN injected at the indicated time after DC priming and analyzed 6 days later. Representative of 2 independent experiments with 2 mice per group for each one. (E) Functional avidity measurement in vivo. Naive (106) or memory (2 × 106) CD45.1 CFSE-labeled Marilyn cells were injected intravenously into B6 mice, which were then primed by injection into the footpad of 106 LPS-matured DCs loaded with the indicated amount of H-Y peptide. Five days later, the number of naive and memory T cells recovered in the DLN was measured.

Ag-experienced T-cell proliferation is preferentially inhibited during on ongoing immune response. (A) Two-cohort experimental design. A first cohort of 106 naive CD45.2 CFSE-labeled Marilyn cells was injected intravenously into CD45.2 B6 mice that were immunized by injection of 106 H-Y peptide–loaded LPS-matured DCs into the footpad the next day. After a variable (n) number of days, a second cohort of either 106 naive, 5 × 106 effector, or 2 × 106 memory CD45.1 CFSE-labeled Marilyn cells was injected. CFSE profiles of gated TCR+CD45.1+ cells from the popliteal and inguinal lymph nodes draining the immunization site (DLN) were assessed 6 days after transfer of the second cohort. (B) Kinetics of the response of transferred Marilyn TCR Tg T cells. Naive (106) or memory (2 × 106) CD45.1 CFSE-labeled Marilyn cells were injected intravenously into B6 mice that were primed the next day by injection of 106 H-Y peptide–loaded LPS-matured DCs into the footpad. The percentage of transferred T cells (CD45.1+TCR+) in the draining lymph node was measured at the indicated time points and compared with the percentage measured at day 1 in the absence of antigen. Each dot represents one mouse. Pooled data from 2 independent experiments. (C) Stronger inhibition of Ag-experienced versus naive T-cell proliferation. Naive (left panels), effector (middle panels), or memory (right panels) Marilyn T cells were injected into primed B6 hosts in the absence (top panels) or the presence (bottom panels) of a previous cohort of responding T cells injected 6 days earlier, as in panel A. Dot plots of gated TCR+CD45.1+ cells from the DLN are representative of at least 3 experiments with 2 mice each per group. The percentage of undivided cells, among total Tg Marilyn T cells, is indicated. (D) Antigen persists in vivo at least 18 days. H-Y peptide–loaded LPS-matured DCs were injected into the footpad of female B6 mice, previously injected (right panels) or not (left panels) with naive CD45.2 CFSE-labeled Marilyn LN cells. At the indicated time points, naive CD45.1 CFSE-labeled Marilyn LN cells were transferred intravenously. Dot plots of gated TCR+CD45.1+ cells from the DLN injected at the indicated time after DC priming and analyzed 6 days later. Representative of 2 independent experiments with 2 mice per group for each one. (E) Functional avidity measurement in vivo. Naive (106) or memory (2 × 106) CD45.1 CFSE-labeled Marilyn cells were injected intravenously into B6 mice, which were then primed by injection into the footpad of 106 LPS-matured DCs loaded with the indicated amount of H-Y peptide. Five days later, the number of naive and memory T cells recovered in the DLN was measured.
When transferred 1 day before priming, both naive and memory T cells proliferated extensively, then decreased in number (Figure 1B). This proliferation was Ag specific, as there was no response to DCs not bearing H-Y peptide (Figure S2B and Joncker et al22 ). As expected, the memory cells peaked 1 to 2 days earlier than naive cells, and returned to their original numbers, whereas naive cells returned to levels somewhat higher than the input number.
During a typical response, early arriving cells are stimulated, divide, and then migrate to B-cell follicles or leave the node to pursue their effector function. Both new and recirculating cells can also arrive at later times. To track the fate of later arriving T cells, which might arrive when the Ag-specific cell numbers are contracting, we injected a second cohort of naive or memory CFSE-labeled T cells 6 days after priming and studied their phenotype at day 12 (Figure 1C). In the absence of a first cohort of responding T cells (where the low frequency of host H-Y–specific CD4 T cells are likely to be expanding), naive, effector, and memory T cells all responded (Figure 1C top panels). However, the presence of a first cohort (Figure 1C bottom panels) radically changed the situation. The late-arriving naive T cells proliferated less (56% of the cells did not divide at all, compared with 21% in the absence of a first cohort), and memory and effector T cells were almost completely inhibited. Thus, the presence of a first cohort of Tg CD4 T cells had a major effect on the proliferation of CD4 T cells that arrived later, especially on effector and memory cells (even if the naive and memory cells were transferred together and stimulated in the same LN; Figure S3). Why were the late-arriving cells responding less well?
The simplest possibility was that they had run out of Ag. LPS-activated DCs are thought to last only a few days.23 Thus there might have been a rapid decay of Ag to a level too low to sustain T-cell proliferation. To test this, we examined the kinetics of available MHC-II/H-Y peptide complexes by transferring new naive CD45.1 Marilyn cells at different time points after the injection of DCs, and measuring their proliferation. Figure 1D shows that the Ag persisted at least 18 days, even when the frequency of responding T cells was increased at day 0 by injecting 106 Marilyn cells.
Another possibility was that our memory populations were somehow inexplicably less responsive to Ag than the naive cells. Figure 1E shows that this was not the case, as naive and memory cells responded similarly in vivo to DCs loaded with a titration of peptide doses.
Physiologic numbers of endogenous T cells inhibit memory T-cell proliferation
It has been suggested that low proliferative responses can be artifacts of nonphysiologically large numbers of Ag-specific cells resulting from adoptive transfer of TCR-Tg T cells.11,13 To check whether the lower proliferation of late-arriving cells was due to the large number (106) of first-cohort Marilyn cells injected at day 0, we compared the responses of naive and memory Marilyn cells injected at different times into normal B6 female hosts, which have very low frequencies of endogenous CD4 T cells specific for H-Y. The patterns were the same. Marilyn cells injected at time 0 proliferated extensively, most of them dividing more than 6 to 7 times, and completely diluting the CFSE dye (Figure 2Ai,ii), but cells injected at day 6 proliferated much less, and once again the memory cells divided fewer times than the naive cells (Figure 2Aiii,iv). To lower the endogenous frequency of H-Y–specific cells even further, we used OT-II hosts on a normal B6 background, which have sufficiently large numbers of T cells in the periphery to prevent space-induced expansion but, because 95% of their CD4 T cells are specific for ovalbumin, have a 20-fold lower frequency of anti–H-Y T cells compared with normal B6 females. In these hosts, both naive and memory Marilyn T cells injected at day 6 proliferated better than in normal B6 hosts, although not as well as when injected at day 0 (Figure 2Avii,viii). To create a physiologic increase in the endogenous frequency, we mimicked a previously vaccinated host by priming B6 females with male splenocytes 1 month before challenging them with H-Y–loaded DCs. Six days later, we injected the mice with naive or memory Marilyn cells, and found that the response was weaker than in unprimed hosts (Figure 2Av,vi). Once again, memory cells proliferated less than naive cells, with very few undergoing more than one division.
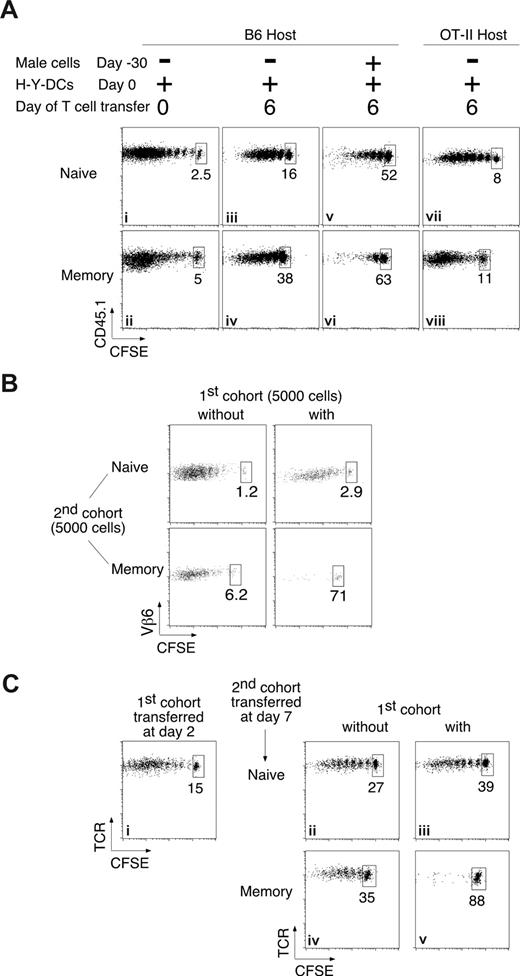
The preferential inhibition of memory T cells is observed under physiological conditions. (A) Physiologic numbers of responding endogenous polyclonal T cells preferentially inhibit memory T-cell proliferation. Naive (106; top panels) or memory (2 × 106; bottom panels) CD45.1 CFSE-labeled Marilyn cells were injected intravenously into B6 (i-vi) or OT-II (vii,viii) mice immunized by injection of 106 H-Y peptide–loaded LPS-matured DCs into the footpad. (i,ii) Low initial frequencies of endogenous responding T cells do not inhibit naive or memory Marilyn T cells; naive or memory CD45.1 Marilyn T cells were injected on the same day as the H-Y peptide–loaded DCs. (iii-vi) Higher frequencies occurring later in the response inhibit proliferation of newly entering cells. Six days after the initiation of the primary endogenous response by injection of peptide-pulsed DCs into naive B6 hosts (iii,iv), or after a boost of mice that had been immunized 30 days previously (v,vi), new naive or memory CD45.1 CFSE-labeled Marilyn cells were injected, and proliferation was analyzed 6 days later. (vii,viii) Extremely low initial frequencies do not expand to inhibitory numbers in 6 days. Marilyn T cells were injected and analyzed as in panels iii,iv, but into OT-II hosts. Plots are of gated TCR+CD45.1+ cells from the DLN, and are representative of at least 2 experiments with at least 2 mice each per group. (B) Small numbers of responding T cells strongly inhibit small numbers of memory T cells. Five thousand naive (top panels) or memory (bottom panels) CD45.1 Marilyn T cells were injected into primed B6 hosts in the absence (left panels) or the presence (right panels) of a previous cohort of 5000 CD45.2 Marilyn T cells injected 6 days earlier, as in Figure 1A. Pooled draining lymph nodes of 8 to 20 mice were studied. Dot plots of gated TCR+CD45.1+ cells with the percentage of undivided cells are shown. (C) An immune response to endogenous DCs that have processed Ag from a peripheral solid tumor preferentially inhibits memory T-cell proliferation. CD45.2 B6 mice received a transplant of an MCA101 tumor cell line, transfected with the DBY protein.24 Two days later, they were given a first cohort of CD45.2+ Marilyn T cells (right panels) or not (left panels). Naive (top panels) or memory (bottom panels) CD45.1+ Marilyn T cells were injected 5 days later. Dot plots of gated TCR+CD45.1+ cells from the immunization DLN are representative of at least 3 experiments with 2 mice each per group.

The preferential inhibition of memory T cells is observed under physiological conditions. (A) Physiologic numbers of responding endogenous polyclonal T cells preferentially inhibit memory T-cell proliferation. Naive (106; top panels) or memory (2 × 106; bottom panels) CD45.1 CFSE-labeled Marilyn cells were injected intravenously into B6 (i-vi) or OT-II (vii,viii) mice immunized by injection of 106 H-Y peptide–loaded LPS-matured DCs into the footpad. (i,ii) Low initial frequencies of endogenous responding T cells do not inhibit naive or memory Marilyn T cells; naive or memory CD45.1 Marilyn T cells were injected on the same day as the H-Y peptide–loaded DCs. (iii-vi) Higher frequencies occurring later in the response inhibit proliferation of newly entering cells. Six days after the initiation of the primary endogenous response by injection of peptide-pulsed DCs into naive B6 hosts (iii,iv), or after a boost of mice that had been immunized 30 days previously (v,vi), new naive or memory CD45.1 CFSE-labeled Marilyn cells were injected, and proliferation was analyzed 6 days later. (vii,viii) Extremely low initial frequencies do not expand to inhibitory numbers in 6 days. Marilyn T cells were injected and analyzed as in panels iii,iv, but into OT-II hosts. Plots are of gated TCR+CD45.1+ cells from the DLN, and are representative of at least 2 experiments with at least 2 mice each per group. (B) Small numbers of responding T cells strongly inhibit small numbers of memory T cells. Five thousand naive (top panels) or memory (bottom panels) CD45.1 Marilyn T cells were injected into primed B6 hosts in the absence (left panels) or the presence (right panels) of a previous cohort of 5000 CD45.2 Marilyn T cells injected 6 days earlier, as in Figure 1A. Pooled draining lymph nodes of 8 to 20 mice were studied. Dot plots of gated TCR+CD45.1+ cells with the percentage of undivided cells are shown. (C) An immune response to endogenous DCs that have processed Ag from a peripheral solid tumor preferentially inhibits memory T-cell proliferation. CD45.2 B6 mice received a transplant of an MCA101 tumor cell line, transfected with the DBY protein.24 Two days later, they were given a first cohort of CD45.2+ Marilyn T cells (right panels) or not (left panels). Naive (top panels) or memory (bottom panels) CD45.1+ Marilyn T cells were injected 5 days later. Dot plots of gated TCR+CD45.1+ cells from the immunization DLN are representative of at least 3 experiments with 2 mice each per group.
Physiologic numbers of Tg T cells preferentially inhibit memory T-cell proliferation
Having checked for the effect of physiologic numbers in the first cohort, we turned to the second. We decreased the number of responding T cells by 200-fold, transferring only 5000 cells in the first and second cohorts to generate a specific T-cell frequency of approximately 0.001%. We injected the second cohorts of T cells 6 days after priming and examined their proliferation pattern 8, instead of 6, days later, to account for the smaller inoculum size. In the absence of the first cohort, both naive and memory cells proliferated extensively, only 1% to 6% of cells remained undivided (Figure 2B left panels), but once again the presence of a first cohort preferentially inhibited the memory cells (Figure 2B right panels). Thus, the preferential inhibition of memory T-cell proliferation is observed with cell frequencies within the physiologic range.
The preferential inhibition of Ag-experienced T-cell proliferation occurs with endogenous APCs
Another potentially nonphysiologic aspect of our studies concerned the use of bone marrow–derived DCs, which may not represent any normal in vivo DC population. We therefore designed an experiment where the transferred T cells would encounter Ag on normal endogenous APCs. We used an MCA101 tumor cell line, transfected with DBY-H-Y,25 which cannot directly present to Marilyn T cells because it lacks MHC class II molecules. When the tumor is given to B6 recipients, the H-Y antigen is captured and represented by normal endogenous APCs. Two days after tumor inoculation, we transferred a first cohort of naive CD45.2 Marilyn cells and, 5 days later, a second cohort of naive or memory CD45.1 Marilyn cells. The initial cohort of Marilyn cells proliferated extensively (Figure 2C), but, as before, the second cohort was inhibited, especially the memory cells (88% undivided memory vs 39% undivided naive cells). Overall, therefore, the inhibition of proliferation due to an ongoing immune response seems to be a physiologic effect produced by normal endogenous T cells and normal endogenous APCs.
Inhibition requires the continued presence of the first cohort of T cells
To determine the mechanism by which memory T cells are preferentially inhibited, we began by asking if the early-arriving T cells must be continuously present. As a first cohort, we injected Marilyn T cells expressing a DTR (A.K. and B.M., manuscript in preparation) into OT-II recipients (to minimize H-Y–specific host T cells, which would be resistant to DT), primed them 1 day later with H-Y-DCs, and killed them 5 days after that with an injection of DT, which induced approximately 90% depletion of the first cohort. On day 6, we injected a second cohort of CFSE-labeled naive or memory Marilyn cells. As usual, both naive and memory T cells proliferated well in the absence of a first cohort (Figure 3Ai,ii), whereas the presence of a first cohort moderately inhibited the naive cells (Figure 3Aiii) and severely inhibited the memory cells (Figure 3Aiv). Removing the first cohort a day before transfer of the second cohort almost completely restored the proliferation of both the naive and memory inocula (Figure 3Av,vi). Thus, although some Ag is certainly stripped from the APCs by the early-responding T cells,26 the release of inhibition when the early-responding T cells are removed suggests that decreases in APC-associated Ag cannot alone account for the inhibition of proliferation of later-arriving memory T cells.
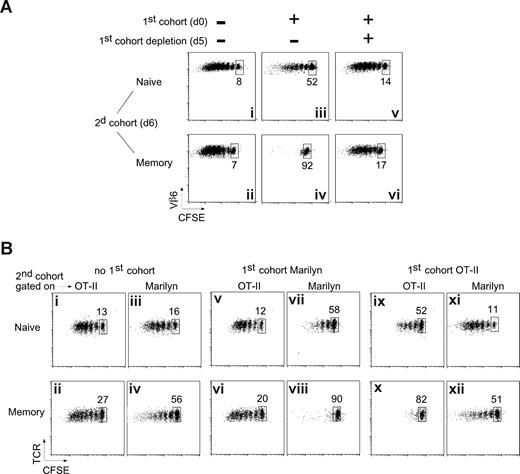
The inhibition is Ag specific and requires the presence of the first cohort of T cells. (A) The inhibition depends on the presence of the first cohort of T cells. Naive (106) or memory (2 × 106) Marilyn T cells were injected into OT-II hosts that had been primed 6 days earlier with H-Y peptide–loaded mature DCs, in the presence (iii-vi) or not (i,ii) of a first cohort of Marilyn-Lat-DTR T cells. The day before the injection of the second cohort, Marilyn-Lat-DTR cells were deleted (v,vi) or not (iii,iv) by the injection of DT. Dot plots of gated CD4+CD45.1+ cells from the DLN studied 6 days later (day 6 + 6) are representative of at least 2 experiments with 2 mice each per group. (B) The inhibition is Ag specific. CD45.1 B6 mice were injected or not (i-iv) with a first cohort of naive CFSE-labeled Marilyn (v-viii) or OT-II (ix-xii) Tg CD4 T cells and were then immunized with LPS-matured DCs pulsed with both OVA and H-Y peptides. Six days later, CFSE-labeled naive (top panels) or memory (bottom panels) OT-II and Marilyn cells were coinjected in the same mouse. Proliferation was measured on the second cohorts (CD45.2+, and Vβ6+ for Marilyn, Vα2hi for OT-II) in the same mouse 6 days later. Representative of 2 independent experiments with 2 mice each per group.

The inhibition is Ag specific and requires the presence of the first cohort of T cells. (A) The inhibition depends on the presence of the first cohort of T cells. Naive (106) or memory (2 × 106) Marilyn T cells were injected into OT-II hosts that had been primed 6 days earlier with H-Y peptide–loaded mature DCs, in the presence (iii-vi) or not (i,ii) of a first cohort of Marilyn-Lat-DTR T cells. The day before the injection of the second cohort, Marilyn-Lat-DTR cells were deleted (v,vi) or not (iii,iv) by the injection of DT. Dot plots of gated CD4+CD45.1+ cells from the DLN studied 6 days later (day 6 + 6) are representative of at least 2 experiments with 2 mice each per group. (B) The inhibition is Ag specific. CD45.1 B6 mice were injected or not (i-iv) with a first cohort of naive CFSE-labeled Marilyn (v-viii) or OT-II (ix-xii) Tg CD4 T cells and were then immunized with LPS-matured DCs pulsed with both OVA and H-Y peptides. Six days later, CFSE-labeled naive (top panels) or memory (bottom panels) OT-II and Marilyn cells were coinjected in the same mouse. Proliferation was measured on the second cohorts (CD45.2+, and Vβ6+ for Marilyn, Vα2hi for OT-II) in the same mouse 6 days later. Representative of 2 independent experiments with 2 mice each per group.
The inhibition is Ag specific
It has been shown in several systems that CD4 T cells can influence other T cells by educating,27 licensing,28,29 suppressing,10,30 or otherwise modifying7 the APCs they bind to. In these cases, the Ag specificity of the “educator” T cell needs not be the same as that of the T cell that is ultimately influenced. To determine whether the early-responding T cells inhibit late-arriving memory T cells by modifying the APCs, we asked whether the effect was Ag specific. We added a second TCR-Tg T cell with a different Ag specificity but the same restriction element: OT-II (specific for OVA/Ab). We transferred either Marilyn or OT-II into CD45.1 B6 hosts, then immunized with DCs loaded with both OVA and H-Y peptides. After 6 days, we transferred a mixture of CFSE-labeled Marilyn and OT-II cells (both naive or both memory) and analyzed them 6,days later, gating for CD45.2-Vβ6+ (Marilyn) or CD45.2-Vα2hi (OT-II).
If the inhibitory effects were mediated by DC modifications, or by soluble factors, then ongoing responses to either OVA or H-Y should inhibit both the OT-II and Marilyn T cells in the second cohort. However, this was not the case; a first cohort of Marilyn T cells had no effect on OT-II cells (Figure 3Bv,vi compared with 3Bi,ii), although it inhibited the proliferation of a second cohort of Marilyn cells (Figure 3Bvi-viii and 3Biii,iv). Similarly, a first cohort of OT-II T cells (Figure 3Bix-xii) inhibited proliferation of OT-II but not of Marilyn cells. As before, memory T cells were inhibited more strongly (Figure 3Bviii,x). These results show that the inhibitory effect of early-responding T cells is (1) generalizable to more than one T-cell clone; (2) not due to global modification of the DCs or the LN environment, and (3) not due to interclonal competition. Finally, in the absence of a first cohort, memory T cells can respond to the APCs, showing that the preferential inhibition of memory T cells is not due to an inability to functionally encounter the APCs.
Activated CD4 T cells capture and present MHC-II in vivo during an ongoing immune response
Although known for decades,31,32 it was recently rediscovered that T cells can specifically acquire MHC/peptide complexes from the cells with which they interact.26,33-35 In vitro, Ag presentation by T cells can induce activation of naive T cells versus anergy of activated T cells.35 In vivo, both inhibition36 and activation37 have been seen. If the presentation of captured complexes by T cells resulted in an inhibition of proliferation, one would see exactly the sort of inhibition that we had seen.
We therefore asked whether early-responding Marilyn T cells might acquire MHC-II molecules. We injected naive CD45.1 Marilyn T cells into CD45.2 recipients 20 hours before injecting H-Y-DCs carrying a GFP fusion protein of MHC-II-Ab.21 By day 2, the activated Marilyn T cells had captured a considerable amount of MHC-II from the DCs (Figure 4Ai) and were displaying these captured molecules on the cell surface, as evidenced by the staining with anti-Ab monoclonal antibody (Figure 4Aiii,iv), although approximately 50-fold less per cell than did the DCs (Figure 4Av), measured by GFP mean fluorescence (MFI). The T cells did not pick up GFP-MHC-II molecules in the contralateral LN (Figure 4Aii,iv), to which the injected DCs did not travel (Figure 4Avi).
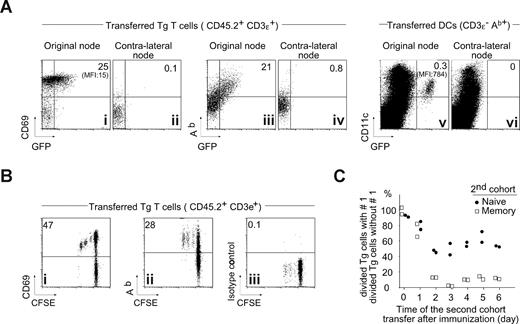
Activated T cells acquire MHC-II molecule during the ongoing immune response in vivo. (A) Acquisition of MHC-II molecules by activated T cells in vivo. Naive Tg CD45.1 Marilyn cells (106) were injected intravenously into B6 mice that were immunized by injection of 106 H-Y peptide–loaded Ab-GFP knockin DCs21 into the footpad. Two days later, CD69, Ab, and GFP expression were analyzed on the transferred T cells in the draining LN and the nondraining LN (CD45.1+TCR+) by 5-color FACS with doublet exclusion. (B) Proliferating T cells acquire MHC-II in vivo. Naive CFSE-labeled Tg CD45.1 Marilyn cells (106) were injected intravenously into B6 mice and immunized as in panel A. Two days later, CD69 and Ab expression was analyzed in the draining LN on the transferred T cells (CD45.1+TCR+) by 5-color FACS with doublet exclusion. (C) The inhibition starts at day 1 and plateaus by day 2. H-Y peptide–loaded DCs (106) were injected into the footpad of female B6 mice that contained (or not) a first cohort of 106 naive CD45.2 CFSE-labeled Marilyn LN cells. At the indicated time points, a second cohort of 106 CD45.1 CFSE-labeled naive Marilyn LN or 2 × 106 memory Marilyn T cells was injected, and the CFSE profile assessed 6 days later. The percentage of divided TCR+CD45.1+ Marilyn cells in the second cohort is shown for cells in the presence of a first cohort relative to the percentage seen in its absence. Each point is an individual mouse.

Activated T cells acquire MHC-II molecule during the ongoing immune response in vivo. (A) Acquisition of MHC-II molecules by activated T cells in vivo. Naive Tg CD45.1 Marilyn cells (106) were injected intravenously into B6 mice that were immunized by injection of 106 H-Y peptide–loaded Ab-GFP knockin DCs21 into the footpad. Two days later, CD69, Ab, and GFP expression were analyzed on the transferred T cells in the draining LN and the nondraining LN (CD45.1+TCR+) by 5-color FACS with doublet exclusion. (B) Proliferating T cells acquire MHC-II in vivo. Naive CFSE-labeled Tg CD45.1 Marilyn cells (106) were injected intravenously into B6 mice and immunized as in panel A. Two days later, CD69 and Ab expression was analyzed in the draining LN on the transferred T cells (CD45.1+TCR+) by 5-color FACS with doublet exclusion. (C) The inhibition starts at day 1 and plateaus by day 2. H-Y peptide–loaded DCs (106) were injected into the footpad of female B6 mice that contained (or not) a first cohort of 106 naive CD45.2 CFSE-labeled Marilyn LN cells. At the indicated time points, a second cohort of 106 CD45.1 CFSE-labeled naive Marilyn LN or 2 × 106 memory Marilyn T cells was injected, and the CFSE profile assessed 6 days later. The percentage of divided TCR+CD45.1+ Marilyn cells in the second cohort is shown for cells in the presence of a first cohort relative to the percentage seen in its absence. Each point is an individual mouse.
To determine whether the T cells displaying MHC-II were those that had been recently activated, we injected CFSE-labeled CD45.1 Marilyn cells into CD45.2 hosts 20 hours before injecting WT H-Y-DCs. Two days later, all the activated Marilyn T cells displayed MHC-II molecules (Figure 4Bii). This suggested that, if capture and presentation of H-Y by Marilyn cells were the mechanism of inhibition, the inhibitory effect should be apparent very early in the response. To test this, we injected a second cohort of naive or memory Marilyn T cells at various times after initiating the primary immune response (Figure 4C). The inhibition of memory T-cell proliferation reached a maximum as soon as day 2. Thus far, these results indicate that responding CD4 T cells can capture MHC-II complexes during a primary immune response in vivo, and this capture correlates kinetically with the inhibition of memory T-cell recruitment.
Activated Marilyn CD4 T cells can capture and present MHC-II/peptide complexes in vitro
To test directly whether Marilyn T cells can capture and present H-Y peptides and inhibit memory T-cell proliferation, we first established an in vitro model. We incubated naive Marilyn cells overnight with H-Y peptide–loaded CFSE-labeled, LPS-matured BMDCs (H-Y-DCs), and then purified the T cells by FACS. Less than 0.02% contaminating DCs remained (Figure 5Ai), and most of these T cells expressed MHC-II molecules (“H-Y T cells,” Figure 5Aiii). We then fixed the purified H-Y T cells to prevent any chemokine or cytokine secretion that might induce Ag-independent T-cell proliferation38 and used them as APCs for naive or memory CFSE-labeled Marilyn T cells. We measured CD69 expression (Figure 5B), at 24 hours as an early marker of T-cell activation, and proliferation at 3 days (Figure 5C). The captured MHC-II complexes did indeed contain the H-Y Ag, as the H-Y T cells stimulated both naive and memory Marilyn cells to up-regulate CD69 (Figure 5Biii,iv). Anti–MHC-II antibodies blocked this activation (Figure S4A), showing that the presentation was MHC dependent. As controls, we used naive Marilyn T cells that had not encountered H-Y-DCs (Figure 5Bi-ii), or OT-II T cells that had captured MHC-II/OVA peptide complexes (Figure S4B), both of which were nonstimulatory.
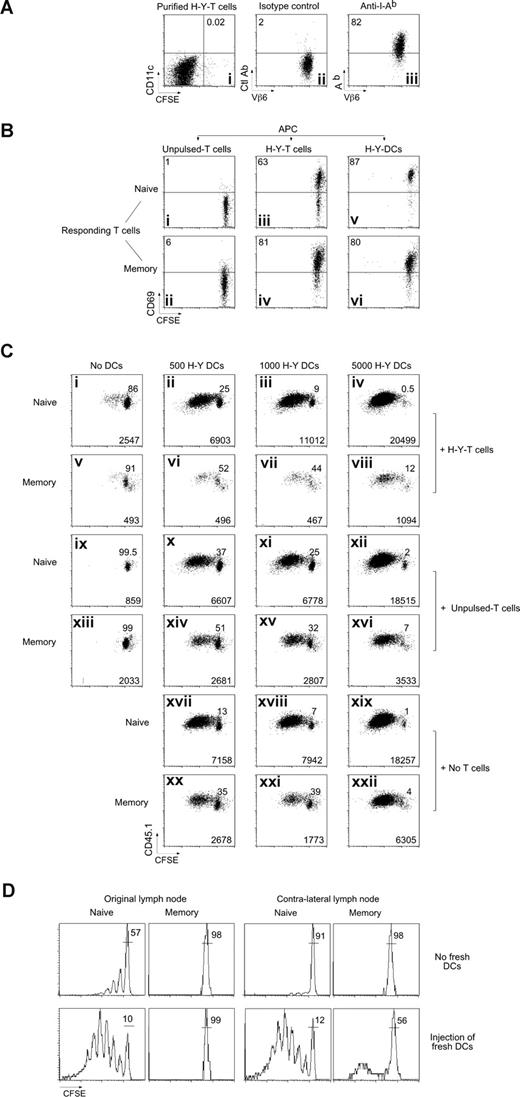
Marilyn CD4 T cells capture MHC-II/H-Y in vitro, and induce death of memory but not naive T cells. (A) Purification of MHC-II–bearing T cells. Lymph node CD45.2 Marilyn cells were incubated with H-Y peptide–loaded (100 nM), CFSE-labeled, LPS-matured DCs. Twenty hours later, the T cells were FACS sorted according to CD11c and CFSE expression with exclusion of the doublets. (i) Postsort analysis of the CFSEneg/CD11cneg T-cell fraction. (ii,iii) Expression of Ab by the sorted T cells. (B) T cells that have captured MHC-II/H-Y complexes present them to naive and memory Marilyn T cells. (i,ii) CD45.2 fixed (as described in “In vitro experiments”) naive LN Marilyn cells (0.5 × 106), (iii,iv) 0.5 × 106 purified CD45.2 fixed T cells that had captured MHC-II/H-Y complexes, or (v,vi) 5000 purified H-Y–bearing fresh DCs were used to stimulate 2 × 104 CFSE-labeled CD45.1 naive Marilyn LN cells or negatively purified memory Marilyn T cells. CD69 expression was measured the following day. Representative of 3 experiments. (C) T cells that have captured MHC-II/H-Y complexes suppress memory but not naive Marilyn T-cell proliferation. Purified CD45.2 fixed naive LN Marilyn cells (0.5 × 106), or 0.5 × 106 purified CD45.2 fixed T cells that had captured MHC-II/H-Y complexes (H-Y T cells), or not (unpulsed T cells), and/or various numbers of H-Y–bearing fresh DCs were used to stimulate 2 × 104 CD45.1 naive or memory Marilyn T cells that had been negatively purified by FACS and then CFSE labeled. Proliferation was measured at day 3 by CFSE dilution. Percentage of undivided cells is indicated in each panel, and numbers of recovered cells/well are indicated at the bottom of each panel. Representative of 4 experiments. (D) Fresh DCs, able to stimulate naive cells, cannot relieve inhibition of memory T cells. B6 mice were injected with CD45.2 CFSE-labeled Marilyn cells and were immunized the next day with H-Y peptide–loaded LPS-matured DCs in one hind footpad. Naive or memory CD45.1 CFSE-labeled Marilyn cells were transferred 5 days later. Four hours later, some of the mice (bottom panels) received a second immunization with H-Y peptide–loaded DCs into both the original and the contralateral footpad. The CFSE profile is shown on the second cohort (TCR+ CD45.1+ cells) in the original DLN (left panels) or in the contralateral DLN (right panels) 6 days after they were injected. Representative of 2 experiments with 2 mice per group in each.

Marilyn CD4 T cells capture MHC-II/H-Y in vitro, and induce death of memory but not naive T cells. (A) Purification of MHC-II–bearing T cells. Lymph node CD45.2 Marilyn cells were incubated with H-Y peptide–loaded (100 nM), CFSE-labeled, LPS-matured DCs. Twenty hours later, the T cells were FACS sorted according to CD11c and CFSE expression with exclusion of the doublets. (i) Postsort analysis of the CFSEneg/CD11cneg T-cell fraction. (ii,iii) Expression of Ab by the sorted T cells. (B) T cells that have captured MHC-II/H-Y complexes present them to naive and memory Marilyn T cells. (i,ii) CD45.2 fixed (as described in “In vitro experiments”) naive LN Marilyn cells (0.5 × 106), (iii,iv) 0.5 × 106 purified CD45.2 fixed T cells that had captured MHC-II/H-Y complexes, or (v,vi) 5000 purified H-Y–bearing fresh DCs were used to stimulate 2 × 104 CFSE-labeled CD45.1 naive Marilyn LN cells or negatively purified memory Marilyn T cells. CD69 expression was measured the following day. Representative of 3 experiments. (C) T cells that have captured MHC-II/H-Y complexes suppress memory but not naive Marilyn T-cell proliferation. Purified CD45.2 fixed naive LN Marilyn cells (0.5 × 106), or 0.5 × 106 purified CD45.2 fixed T cells that had captured MHC-II/H-Y complexes (H-Y T cells), or not (unpulsed T cells), and/or various numbers of H-Y–bearing fresh DCs were used to stimulate 2 × 104 CD45.1 naive or memory Marilyn T cells that had been negatively purified by FACS and then CFSE labeled. Proliferation was measured at day 3 by CFSE dilution. Percentage of undivided cells is indicated in each panel, and numbers of recovered cells/well are indicated at the bottom of each panel. Representative of 4 experiments. (D) Fresh DCs, able to stimulate naive cells, cannot relieve inhibition of memory T cells. B6 mice were injected with CD45.2 CFSE-labeled Marilyn cells and were immunized the next day with H-Y peptide–loaded LPS-matured DCs in one hind footpad. Naive or memory CD45.1 CFSE-labeled Marilyn cells were transferred 5 days later. Four hours later, some of the mice (bottom panels) received a second immunization with H-Y peptide–loaded DCs into both the original and the contralateral footpad. The CFSE profile is shown on the second cohort (TCR+ CD45.1+ cells) in the original DLN (left panels) or in the contralateral DLN (right panels) 6 days after they were injected. Representative of 2 experiments with 2 mice per group in each.
Although the early induction of CD69 expression was similar for naive and memory T-cell responders, the consequences were different. For naive responders, stimulation by Ag-bearing T cells did not lead to efficient day-3 proliferation, although it prevented death (Figure 5Ci vs 5Cix), and allowed them later to respond to H-Y-DCs (Figure 5Cii-iv). In contrast, memory T cells were strongly inhibited, and died (Figure 5Cv vs 5Cxiii) even in the presence of additional H-Y-DCs (Figure 5Cvi-viii vs Figure 5Cxx-xxii). The suppression depended on specific Ag presentation, as it was not induced by T cells that had not captured the H-Y Ag (unpulsed T cells; Figure 5Cxiv-xvi). Thus, in vitro, Ag-bearing T cells compete with Ag-loaded DCs and inhibit proliferation of memory CD4 T cells.
To determine whether the dominant inhibition over proliferation induced by new H-Y–loaded DCs is also true in vivo, we set up an ongoing immune response with a first cohort of T cells plus peptide-loaded DCs, and then injected fresh peptide-pulsed DCs into the original footpad, or into the contralateral foot at the same time as the second cohort of naive or memory Marilyn cells. Although the new H-Y-DCs rescued most of the proliferative capacity of late-arriving naive T cells (Figure 5D), they did not rescue memory cells, either in the original or in the distant node. Thus, the inhibitory effect dominates both in vitro and in vivo, and as expected for a T cell–mediated effect, it also circulates to distant nodes, although with a slight delay, allowing a few cells to escape for a small number of divisions.
Model for the regulation of CD4 T-cell expansion during an immune response
Figure 6 shows one way to account for all the experimental data: (1) At the onset of the immune response, naive CD4 T cells (and also memory cells during secondary responses) are recruited and stimulated by activated Ag-bearing APCs. (2) These CD4 T cells capture their specific MHC-peptide complexes. (3) As the number of CD4 T cells bearing captured MHC-peptide complexes increases, they begin to outnumber the APCs, thereby increasing the probability that newly arriving T cells (and any T cells that have just been activated and need a second hit to continue proliferating8,39 ) will encounter them before meeting a proper professional APC.40 (4) The T-T interaction leads to inhibition of Ag-experienced but not naive T cells.
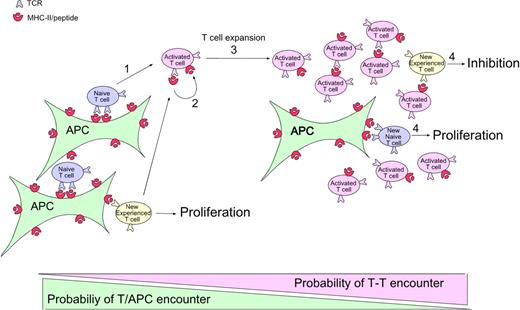
Model of CD4 T-cell autoregulation of an immune response. (1) At the onset of the immune response, naive (and also memory in the case of a secondary response, which is not represented here) CD4 T cells are recruited and stimulated by activated APCs displaying antigen. (2) During activation, these CD4 T cells capture the specific MHC-peptide complexes bound by their TCRs. (3) At later time points, the number of activated CD4 T cells that have captured MHC-peptide complexes increases. As these activated Ag-bearing T cells begin to outnumber the APCs, there is increasing probability that newly arriving T cells (and any T cells that have just been activated and need a second hit to continue to divide) will encounter them before encountering a proper professional APC. (4) This T-T interaction leads to inhibition of the Ag-experienced T cells, whereas the normal interaction of naive cells with the professional APCs leads to proliferation.

Model of CD4 T-cell autoregulation of an immune response. (1) At the onset of the immune response, naive (and also memory in the case of a secondary response, which is not represented here) CD4 T cells are recruited and stimulated by activated APCs displaying antigen. (2) During activation, these CD4 T cells capture the specific MHC-peptide complexes bound by their TCRs. (3) At later time points, the number of activated CD4 T cells that have captured MHC-peptide complexes increases. As these activated Ag-bearing T cells begin to outnumber the APCs, there is increasing probability that newly arriving T cells (and any T cells that have just been activated and need a second hit to continue to divide) will encounter them before encountering a proper professional APC. (4) This T-T interaction leads to inhibition of the Ag-experienced T cells, whereas the normal interaction of naive cells with the professional APCs leads to proliferation.
Direct inhibition of memory T-cell proliferation by Ag-bearing T cells
To directly test the in vivo inhibitory capacity of T cells that have captured their specific Ag, we allowed Marilyn T cells to capture H-Y/MHC complexes from DCs in vitro, as in Figure 5B, sorted them to obtain pure T cells, and transferred them by footpad injection into adoptive hosts into which CFSE-labeled naive or memory Marilyn T cells had been transferred beforehand (Figure 7A). As controls for any potential nonspecific effects of infusing activated T cells, we also used Marilyn cells that had been stimulated with anti-TCR, as these can capture MHC class II (Figure S5A) but not H-Y peptide. After a 6-day period to allow for T-T interactions in vivo, we injected fresh H-Y peptide–loaded DCs into the same footpad. Six days later, we examined T-cell proliferation (Figure 7B). Whereas naive T cells proliferated in all conditions, memory T cells proliferated if they had interacted with anti-TCR–activated Marilyn T cells, and not with H-Y–bearing Marilyn cells. As the number of anti-TCR–activated T cells in the lymph node was approximately 3-fold higher than that of H-Y–activated cells, (Figure S5B) these results demonstrate that it is the presence of MHC-peptide complexes on the activated T cells that inhibits Ag-experienced T-cell proliferation.
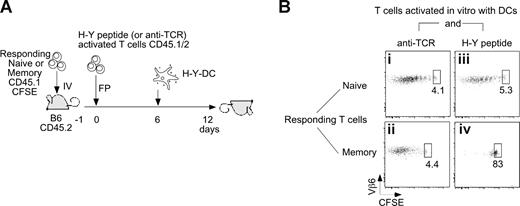
Ag-presenting Marilyn T cells directly inhibit memory T-cell proliferation in vivo. (A,B) Naive (106) or memory (2 × 106) CD45.1 CFSE-labeled Marilyn T cells were injected intravenously into CD45.2 female hosts. In parallel, CD45.1/2 Marilyn T cells were incubated in vitro overnight with CFSE-labeled H-Y peptide–loaded LPS-matured DCs or with DCs and an anti-TCR antibody. The next day, the 2 sets of in vitro–activated Marilyn T cells were FACS sorted according to CFSE and CD11c expression and injected into the footpad. Six days later, H-Y–loaded DCs were injected into the footpad to stimulate the T cells. CFSE patterns were studied in the DLN 6 days later. Dot plots on gated CD45.1+/CD45.2−/Vb6+/CD4+ cells are representative of 2 independent experiments with 2 mice in each.

Ag-presenting Marilyn T cells directly inhibit memory T-cell proliferation in vivo. (A,B) Naive (106) or memory (2 × 106) CD45.1 CFSE-labeled Marilyn T cells were injected intravenously into CD45.2 female hosts. In parallel, CD45.1/2 Marilyn T cells were incubated in vitro overnight with CFSE-labeled H-Y peptide–loaded LPS-matured DCs or with DCs and an anti-TCR antibody. The next day, the 2 sets of in vitro–activated Marilyn T cells were FACS sorted according to CFSE and CD11c expression and injected into the footpad. Six days later, H-Y–loaded DCs were injected into the footpad to stimulate the T cells. CFSE patterns were studied in the DLN 6 days later. Dot plots on gated CD45.1+/CD45.2−/Vb6+/CD4+ cells are representative of 2 independent experiments with 2 mice in each.
Discussion
Altogether the data demonstrate that an ongoing immune response establishes conditions that preferentially inhibit the proliferation of Ag-experienced T cells. The inhibition is Ag specific and occurs very early in the immune response. Although late-arriving naive cells proliferate somewhat less because of some Ag loss from the APCs and because of intraclonal competition, effector/memory cells in the same LN and during the same time period are stopped by an active phenomenon, even in the presence of persisting or newly arriving Ag-presenting APCs.
This is not due to APC education27,41 because the inhibition is Ag specific and allows the DCs to present Ags to other memory T cells (Figure 3B), nor is it due to classical regulatory T cells, whose effects are not Ag specific.10 Neither, for 3 reasons, is this likely to be due to simple competition for Ag.11,12,42 First, competition should be most effective against naive, rather than memory, T cells. Second, the inhibition is set up too quickly (by day 2; Figure 4C), when the original number of proliferating cells is still low. Third, the injection of a new set of Ag-loaded DCs should increase the number of stimulatory APCs sufficiently to overcome the competition. Although it does increase the proliferation of naive CD4 T cells (Figure 5D), it does not for Ag-experienced cells.
All of our data fit with the scenario that the inhibition is due to the capture of MHC/Ag complexes by the responding T cells and presentation of these complexes to new T cells. The capture is clearly measurable by day 2 after the initiation of a primary response (Figure 4A), at which time the inhibition of memory cell proliferation is also clearly visible (Figure 4C). At this time, new T cells entering the lymph node would encounter 2 types of cells bearing their Ag: the APCs that entered from Ag-loaded tissues and the T cells that have captured MHC/Ag complexes from those and earlier APCs (Figure 6).
There are several ways in which experienced CD4 T cells might be preferentially inhibited. First, the Ag-presenting T cells might express inhibitory ligands for which only experienced T cells have receptors. Alternatively, because the Ag-bearing T cells present less Ag than do APCs, effector/memory T cells may more easily recognize them and be inhibited for lack of proper costimulation, or by the presence of general inhibitory signals. Importantly, this inhibition is likely operative on both Ag-experienced T cells newly entering the LN and on any previously activated naive T cells that require another hit to continue dividing and differentiating.8,39
This type of regulation is analogous to the Ag-specific feedback loop that stops B cells from responding once there is enough circulating antibody (a B cell is inhibited if it recognizes Ag already bound by free antibodies whose Fc portions bind to the FcγRIIB on the B-cell surface).24,43,44 Because T cells do not usually secrete their Ag-specific receptors, they need another way to communicate with each other to regulate their numbers. The capture and presentation of Ag is a unique Ag-specific feedback loop that is completely proportional to the number of responding T cells and the number of Ag-presenting APCs. Until the responding T cells begin to outnumber the APCs, proliferation continues. But as APC numbers drop (perhaps signaling the end of the infection) or as responding T cells proliferate (signaling that there are sufficient numbers), proliferation stops. This scenario is compatible with recent results showing an inverse relationship between CD4 cell frequency and proliferation/differentiation.13
Why should the inhibition preferentially target Ag-experienced T cells? There might be several biologic advantages. First, it allows for the continuous generation of a diverse immune repertoire, as new naive CD4 or CD8 T cells can still be recruited into the immune response at late time points and display good effector functions.12,45 Second, it allows for the recruitment of naive T cells able to recognize mutating pathogen epitopes. Third, it may allow a small number of initial responders to expand to a useful number before switching off proliferation and switching on differentiation into fully fledged effectors. Finally, a late-responding T cell needing a second hit to keep proliferating might become an effector cell upon encountering an Ag-bearing APC, or a memory cell upon encountering an Ag-bearing T cell.12
We do not know whether such an Ag-capturing quorum-sensing mechanism might also apply to CD8 T cells, as their activation requirements may be different from those of CD4. For example, CD8 T cells have been reported to be able to divide autonomously after their first encounter with Ag,46-48 whereas CD4 T-cell division requires the continuous presence of Ag.8,39 Perhaps differences in the life span of presented MHC-I versus MHC-II Ag complexes49-51 or the ability of CD8 T cells to kill their APCs7 might lead to the requirement for a negative feedback loop for one population of T cells (CD4 T cells) but not the other (CD8 T cells). Alternatively, a similar negative feedback loop could also be operating for CD8 T cells through fratricidal killing.33
For CD4 T cells, the rapidly occurring preferential inhibition of Ag-experienced T cells appears to be a built-in, Ag-specific, quorum-sensing mechanism operating at the site of the immune response to fine-tune the response intensity to the amount of presented Ag and the number of responding T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank S. Laigneau and N. Froux for managing the mouse colonies; V. Premel for expert technical assistance; Z. Maciorowski and C. Guerin for cell sorting; M. Ingersoll, P. Guermonprez, and A. M. Lennon-Dumenil, S. Amigorena, P. Benaroch, P. Bousso, and M. Albert for discussions. We also thank S. Frioux for innocently asking how naive and memory cells behave if they encounter Ag at the same time.
This work was supported by grants from Inserm, Institut Curie, and Ligue contre le cancer (Paris, France), and by the Intramural Research Program of the National Institutes of Health,NIAID. O.L. is “équipe labellisée de la ligue contre le cancer.” The Lat-DTR mice were developed on Plate-forme RIO-MNG de Marseille-Luminy and supported by Association pour la recherche sur le cancer (ARC; Villejuif, France) and the European Community Network of Excellence (MUGEN Network).
National Institutes of Health
Authorship
Contribution: J.H. and A.J. performed most of the experiments with the help of N.J., I.G., and G.D. for some of them; A.K. and B.M. generated the Lat-DTR mouse; P.M. suggested key experiments and concepts and helped with writing; O.L. designed and supervised the work; and J.H., P.M., and O.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olivier Lantz: Laboratoire d'Immunologie, Institut Curie, Paris, France, 26 rue d'Ulm, 75005, Paris, France; e-mail: olivier.lantz@curie.net.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal