To the editor:
The target organ substantially influences the risk of immune responses to therapeutic proteins in gene therapy. For example, intramuscular administration of an adeno-associated viral (AAV) vector causes robust antibody formation to human coagulation factor IX (hF.IX) in mice, which often is not observed in hepatic gene transfer.1 Our studies have demonstrated immune tolerance induction by the hepatic route, resulting in CD4+ T-cell tolerance and an adaptive CD4+CD25+ regulatory T-cell response.2,3 In contrast, the success of gene transfer to skeletal muscle is limited by a local immune response.4,5
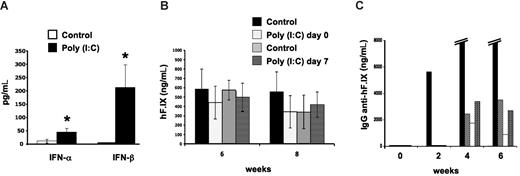
The signals from the tissue to the immune system at the time of gene transfer that determine these organ-specific responses remain unclear. Others have identified Toll-like receptor 3 (TLR3) as one important mechanism of solid organ immune privilege, and activation of TLR3 can break this immunoprivileged state of the liver.6 TLR3 recognizes double-stranded RNA molecules, which may be derived from viral genomes or released from damaged cells, and triggers innate immunity by activation of NF-κB and the production of type I interferons.7 Muscle cells constitutively express TLR3 protein.8 These can be up-regulated upon challenge with inflammatory cytokines, which would explain why inflammatory signals during vector administration cause an immune response to the transgene product in skeletal muscle but not in the liver, where TLR3 is inefficiently activated. To test whether TLR3 signaling plays a role in organ-specific immunity in gene therapy, we injected AAV-hF.IX vectors (identical to those previously used in clinical trials in gene therapy for hemophilia B9,10 ) at a dosage of 4 × 1012 vector genomes/kg into the portal vein of C57BL/6 mice or the muscle of TLR-knockout mice (B6;129S1-Tlr3tm1Flv/J; The Jackson Laboratory, Bar Harbor, ME). Hepatic gene transfer was with or without high-dose polyinosinic-polycytidylic acid [poly (I:C)] (500 μg/animal as described by Lang et al6 ) to activate TLR3 at the time of gene transfer or 7 days later, thereby supplying the presumably missing inflammatory signal. Poly (I:C) treatment caused a rise in systemic IFN-α/β (Figure 1A). However, no antibody response was detectable in poly (I:C)–treated or control animals (data not shown). Both groups showed sustained expression of hF.IX for at least 2 months at comparable levels (Figure 1B). In sharp contrast, intramuscular delivery caused antibodies to hF.IX in 4 of 5 TLR−/− mice at titers indistinguishable from those seen in fully immune-competent mice (peak titers of 1.5-39 μg/mL, Figure 1C, and published4 and unpublished observations, O.C., April 2008), and none of the animals had systemic hF.IX expression (data not shown). Taken together, our studies indicate that TLR3 signaling is not involved in regulation of organ-specific immunity to the transgene product in the context of AAV gene transfer.
Lack of TLR3 signaling in organ-specific immunity to hF.IX. (A) IFN-α/β levels in serum 4 hours after intravenous administration of poly (I:C) compared with control mice (n = 4-5 per group). (B) Plasma levels of hF.IX after hepatic gene transfer with AAV2-ApoE/hAAT-hF.IX vector. C57Bl/6 mice were treated with or without poly (I:C) (control groups) at the time of vector administration or 7 days later (n = 4-5 per group). Note that poly (I:C)–treated animals did not develop antibody response to hF.IX (data not shown). Data in A and B are averages plus or minus SD. *Statistically significant differences (P < .05) in experimental compared with control groups; note that differences in panel B were not significant. (C) Plasma levels of anti-hF.IX IgG in TLR−/− mice after muscle-directed gene transfer (AAV2-CMV-hF.IX vector, n = 5). Each bar represents an individual animal. Four of 5 mice developed antibodies to hF.IX. (One mouse formed very high-titer antibody, 12-39 μg/mL at 4-6 weeks.)
Lack of TLR3 signaling in organ-specific immunity to hF.IX. (A) IFN-α/β levels in serum 4 hours after intravenous administration of poly (I:C) compared with control mice (n = 4-5 per group). (B) Plasma levels of hF.IX after hepatic gene transfer with AAV2-ApoE/hAAT-hF.IX vector. C57Bl/6 mice were treated with or without poly (I:C) (control groups) at the time of vector administration or 7 days later (n = 4-5 per group). Note that poly (I:C)–treated animals did not develop antibody response to hF.IX (data not shown). Data in A and B are averages plus or minus SD. *Statistically significant differences (P < .05) in experimental compared with control groups; note that differences in panel B were not significant. (C) Plasma levels of anti-hF.IX IgG in TLR−/− mice after muscle-directed gene transfer (AAV2-CMV-hF.IX vector, n = 5). Each bar represents an individual animal. Four of 5 mice developed antibodies to hF.IX. (One mouse formed very high-titer antibody, 12-39 μg/mL at 4-6 weeks.)
Authorship
This work was supported by National Institutes of Health grant P01 HL078810 (Project 3) to R.W.H.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roland W. Herzog, University of Florida, Cancer and Genetics Research Center, 1376 Mowry Road, Room 203, Gainesville, FL 32610; e-mail: rherzog@ufl.edu.
References
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal