Abstract
The adult erythron is maintained via dynamic modulation of erythroblast survival potentials. Toward identifying novel regulators of this process, murine splenic erythroblasts at 3 developmental stages were prepared, purified and profiled. Stage-to-stage modulated genes were then functionally categorized, with a focus on apoptotic factors. In parallel with BCL-X and NIX, death-associated protein kinase-2 (DAPK2) was substantially up-modulated during late erythropoiesis. Among hematopoietic lineages, DAPK2 was expressed predominantly in erythroid cells. In a Gata1-IE3.9int-DAPK2 transgenic mouse model, effects on steady-state reticulocyte and red blood cell (RBC) levels were limited. During hemolytic anemia, however, erythropoiesis was markedly deficient. Ex vivo ana-lyses revealed heightened apoptosis due to DAPK2 at a Kit−CD71highTer119− stage, together with a subsequent multifold defect in late-stage Kit−CD71highTer119+ cell formation. In UT7epo cells, siRNA knock-down of DAPK2 enhanced survival due to cytokine withdrawal, and DAPK2's phosphorylation and kinase activity also were erythropoietin (EPO)-modulated. DAPK2 therefore comprises a new candidate attenuator of stress erythropoiesis.
Introduction
Erythroid progenitor cell survival is regulated by unique networked mechanisms. As cell-extrinsic factors, FASL and TRAIL can induce apoptosis, while erythropoietin (EPO) provides essential survival signals via JAK2/STAT5, PI3K/AKT, and RAS/MEK/ERK1,2 routes.1 Cell-intrinsic regulators include BCL-X and NIX, plus GATA1 as a caspase-3 target.2-4 To define new potential key survival-regulating factors, we presently profiled differentially staged primary murine splenic erythroblasts. One discovered erythroid-predominant factor was death-associated protein kinase-2 (DAPK2). Among 3 DAPK serine/threonine kinases,5,6 DAPK1 first was identified as an IFN-γ-induced factor which facilitates cell death initiated by IFN-γ, TNF-α, FAS, or oncogene expression.7 DAPK1 possesses a DAP kinase upper-lobe signature, CaM regulatory domain, ankyrin repeats, and a C-terminal death domain.5,6 ZIPK (zipper-interacting protein kinase/DAPK3/DLK) lacks a CaM domain, but possesses leucine zipper and nuclear localization motifs.5,6,8 DAPK2 retains a related CaM domain, but possesses a unique C-terminal region.5,6,9 Such structural differences further suggest that DAPK1, ZIP-K and DAPK2 likely play unique biologic roles.
DAPK1 can exert proapoptotic effects, potentially via caspase-independent type-II mechanisms.5-7 Gene disruption experiments further place DAPK1 upstream of p53,9 and decreased DAPK1 expression is linked to multiple myeloma, ALL, and colon, breast and lung cancers.10 For DAPK2, overexpression studies in cell lines also point to potential proapoptotic effects,11,12 but to date this has not been investigated in primary cells which normally express, and regulate endogenous DAPK2. Via transcriptome analyses, transgenic mouse experiments, and analyses in EPO-dependent erythroid progenitor cells, we now demonstrate predominant DAPK2 expression in erythroid progenitors; characterize DAPK2's proapoptotic capacity to sharply limit erythropoiesis during hemolytic anemia; and initially associate DAPK2's activity to EPO's actions as a candidate negative feedback factor.
Methods
Transgenic mice and splenic erythroblast preparations
pA2gata1-EE-T-Y343 mice expressing an “EE” tag13 were treated with thiamphenicol for 5 days.14 At 80, 100, or 120 hours after withdrawal, splenocytes were isolated, incubated in 50 U/mL DNase-I, 0.5 U/mL dispase-I, and purified by magnetic-activated cell sorting (MACS).13 For DAPK2 transgenics, a flag-hDAPK2 cDNA was cloned to Gata1-IE3.9int,15 and injected (8 kb Sal-I fragment) into FVB pronuclei. Phenylhydrazine dosing was at 52.5 mg/kg. Blood cell counts were via an ADVIA-120 (Bayer, Tarrytown, NY). Hematocrits and reticulocytes also were assayed by microcentrifugation and thiazole-orange staining.16 For mouse models used herein, all studies and protocols received Institutional Animal Care and Use Committee review and approval from all participating institutions.
Profiling and reverse transcription–qPCR
Primary hematopoietic cells and cell lines
Pre/pro-B cells, granulocytes/monocytes, and mast cells were expanded in BIT9500 medium plus recombinant cytokines as indicated. Erythroblasts were expanded in SP34-EX medium.17-19 NIH-3T3, OP9, G1E/JC4, and EML, UT7epo cells were cultured as detailed in legends.
Flow cytometry and Western blotting
Flow cytometric analyses for KIT, CD71, Ter119, EE-T-Y343, and annexin-V were as described.17-19 Tissues were ground in liquid nitrogen (LN2), and homogenized in an Igepal lysis buffer.17-19 These samples, and cultured cell extracts, then were prepared for Western blotting as previously described.17-19
Lentiviruses
Kinase assays
DAPK2 activity was assayed after immunoprecipitation using myosin light chain (MLC).22 Phospho-Ser318-DAPK2 was assayed via Western blotting.
Results and discussion
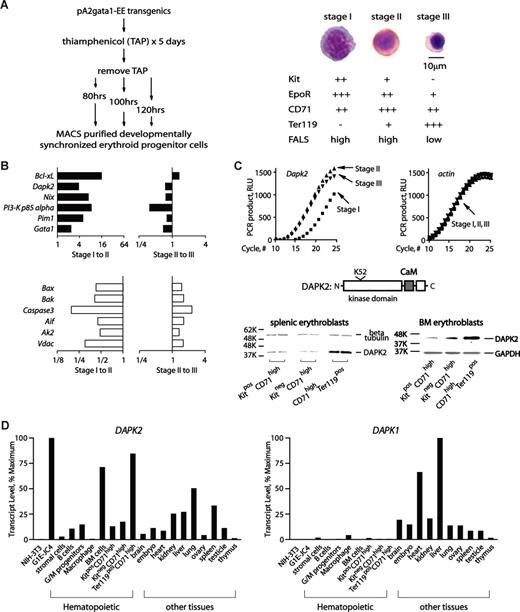
(Pro)erythroblasts at 3 developmental stages first were prepared, and profiled. Specifically, thiamphenicol was used to limit BFUe formation in pA2gata1-EE-T-Y343 mice.13,14 Upon thiamphenicol withdrawal, splenic erythropoiesis initiated synchronously and at 80, 100 and 120 hours, KithighCD71+Ter119−; KitlowCD71+Ter119+; and Kit−CD71+Ter119high cells (stages I, II and III) were generated at high frequencies (Figure 1A). This approach also enabled efficient erythroblast purification via MACS (Figure 1A and Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Transcriptome-based identification of DAPK2 in erythroid progenitor cells, and its stage- and lineage- restricted expression. (A) Preparation, purification and initial characterization of developmentally staged erythroblasts. Splenic erythroblasts from pA2gata1-EE-Y343 mice were prepared at 80, 100 and 120 hours after thiamphenicol treatment and withdrawal (stages I-III), and were purified via MACS. Purity and stagedness were assessed by flow cytometric analyses of EE-positive cells (see Figure S1A), and by morphologic analyses of cytospin preparations. Following staining with HEMA-3 (Fisher Scientific, Hampton, NH), micrographs were acquired using a Zeiss Axioskop 2 microscope (Carl Zeiss, Thornwood, NY) equipped with a 100×/1.3 numeric aperture oil-immersion objective, and a Spotflex model 15.2 camera (Diagnostic Instruments, Sterling Heights, MI). Spot advance version 4.6 (Diagnostic Instruments) and Photoshop version CS2 software (Adobe Systems, San Jose, CA) were used. Stage I cells are KithighCD71+Ter119− high-FALS (forward-angle light scatter) proerythroblasts; stage II cells are KitlowCD71+Ter119pos high-FALS polychromatophilic erythroblasts; and stage III cells are Kit−CD71+Ter119high low-FALS orthochromatic normoblasts. Stage I, II and III cells were also initially characterized based on relative expression levels of Epo receptor (EpoR) transcripts (assayed via Northern blotting and phosphoimaging; data not shown). (B) Profiling of erythroblast stage-modulated survival factors. For survival factors, profiling outcomes are illustrated as stage I-to-II, and II-to-III modulated sets. Top panels (■) are for transcripts with increased expression at stages I-to-II, while transcripts with decreased stage I-to-II expression (□) are graphed in the bottom subpanel. (C) RT-qPCR results for Dapk2 transcript levels in stage I-III erythroblasts also are shown (beta-actin transcript levels were used as an internal control). DAPK2 and its major structural features also are diagrammed. Stage-wise increases in DAPK2 expression also were observed among developmentally staged splenic and bone marrow (BM) erythroblasts as expanded ex vivo,17-19 fractionated as indexed, and analyzed by Western blotting (anti-DAPK2 antibody, Chemicon, Temecula, CA). (D) DAPK2 is expressed predominantly in the erythroid lineage. Dapk2 and Dapk1 transcript levels in mouse cell lines, primary bone marrow–derived hematopoietic progenitors, and select tissues were assayed by reverse-transcription, and quantitative PCR.17,18 Beta-Actin transcript levels were used as internal controls. For Dapk2 and Dapk1, levels were normalized to maximums.
Transcriptome-based identification of DAPK2 in erythroid progenitor cells, and its stage- and lineage- restricted expression. (A) Preparation, purification and initial characterization of developmentally staged erythroblasts. Splenic erythroblasts from pA2gata1-EE-Y343 mice were prepared at 80, 100 and 120 hours after thiamphenicol treatment and withdrawal (stages I-III), and were purified via MACS. Purity and stagedness were assessed by flow cytometric analyses of EE-positive cells (see Figure S1A), and by morphologic analyses of cytospin preparations. Following staining with HEMA-3 (Fisher Scientific, Hampton, NH), micrographs were acquired using a Zeiss Axioskop 2 microscope (Carl Zeiss, Thornwood, NY) equipped with a 100×/1.3 numeric aperture oil-immersion objective, and a Spotflex model 15.2 camera (Diagnostic Instruments, Sterling Heights, MI). Spot advance version 4.6 (Diagnostic Instruments) and Photoshop version CS2 software (Adobe Systems, San Jose, CA) were used. Stage I cells are KithighCD71+Ter119− high-FALS (forward-angle light scatter) proerythroblasts; stage II cells are KitlowCD71+Ter119pos high-FALS polychromatophilic erythroblasts; and stage III cells are Kit−CD71+Ter119high low-FALS orthochromatic normoblasts. Stage I, II and III cells were also initially characterized based on relative expression levels of Epo receptor (EpoR) transcripts (assayed via Northern blotting and phosphoimaging; data not shown). (B) Profiling of erythroblast stage-modulated survival factors. For survival factors, profiling outcomes are illustrated as stage I-to-II, and II-to-III modulated sets. Top panels (■) are for transcripts with increased expression at stages I-to-II, while transcripts with decreased stage I-to-II expression (□) are graphed in the bottom subpanel. (C) RT-qPCR results for Dapk2 transcript levels in stage I-III erythroblasts also are shown (beta-actin transcript levels were used as an internal control). DAPK2 and its major structural features also are diagrammed. Stage-wise increases in DAPK2 expression also were observed among developmentally staged splenic and bone marrow (BM) erythroblasts as expanded ex vivo,17-19 fractionated as indexed, and analyzed by Western blotting (anti-DAPK2 antibody, Chemicon, Temecula, CA). (D) DAPK2 is expressed predominantly in the erythroid lineage. Dapk2 and Dapk1 transcript levels in mouse cell lines, primary bone marrow–derived hematopoietic progenitors, and select tissues were assayed by reverse-transcription, and quantitative PCR.17,18 Beta-Actin transcript levels were used as internal controls. For Dapk2 and Dapk1, levels were normalized to maximums.
Stage I-III erythroblasts next were analyzed for stage-to-stage transcriptome modulations. Robust profiling outcomes (Figure S1B) allowed for functional categorizations. For 2 broad sets, transcription plus chromatin factors and signal transduction plus cell cycle factors, profiles are presented in Figures S2, S3, and serve to further characterize erythroblast stagedness.
Attention next focused on candidate (anti)apoptotic factors. Twelve genes encoding (anti)apoptotic factors were stage-modulated (Figure 1B). At stage II, 6 were up-modulated, including Bcl-xL, Nix, Pim1, p85alpha, Gata1, and Dapk2. In contrast, Bax, Bak, Caspase-3, Aif, Ak-2 and Vdac were down-modulated 2- to 4-fold. With the exception of Dapk2, these factors have been studied previously in primary cell systems and mouse models. Dapk2 therefore was investigated further. RT-qPCR first confirmed high-level expression in maturing splenic erythroblasts, and this also was observed in bone marrow erythroblasts (Figure 1C,D). DAPK2 levels in early, mid and late stage erythroblasts from both the spleens of phenylhydrazine-treated mice, and bone marrow also were analyzed via Western blotting, with similar results. Dapk2 and Dapk1 levels were further analyzed in several hematopoietic lineages, and tissues. As analyzed among hematopoietic cells, Dapk2's expression was selectively high in bone marrow and in late-stage erythroblasts. Dapk1 levels, in contrast, were relatively low in all hematopoietic lineages assayed (Figure 1D). These results, and additional supporting Western blot experiments (Figure S4), suggested possible erythroid-predominant roles for DAPK2.
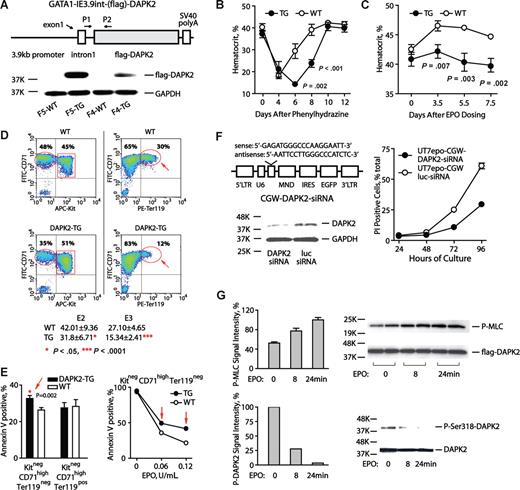
In a transgenic approach, a Gata1-IE3.9int vector15 next was used to reinforce DAPK2 expression (Figure 2A). In Gata1-IE3.9int-DAPK2 mice, peripheral blood cell levels for lymphoid, myeloid, granulocytic lineages were normal (data not shown), and only modest effects on reticulocytes (approximately 1.03% ± 0.30% increase) and hematocrits (approximately 2.33 ± 0.52 point decrease) were observed (Figure S5A). Splenic CD71highTer119+ erythroblasts also were somewhat elevated as 2.75% plus or minus 0.15% of total splenocytes in Gata1-IE3.9int-DAPK2 mice versus 1.25% plus or minus 0.05% in wild-type littermates (Figure S5B). After phenylhydrazine dosing, however, Gata1-IE3.9int-DAPK2 mice exhibited severe anemia. In controls, hematocrits fell to approximately 21.1%, and recovered by approximately day 8. In Gata1-IE3.9int-DAPK2 mice, hematocrits fell to a nadir of approximately 14.3%, and remained comparably low through day 8 (Figure 2B). Furthermore, 30% of Gata1-IE3.9int-DAPK2 mice failed to reverse anemia and died by day 5 (Figure S5C). EPO responses also were examined. In controls, EPO (1000 U/kg) increased hematocrits by 5.6 points. In Gata1-IE3.9int-DAPK2 mice, this response was limited to a 1.7 point increase (ie, 31.3% of control response values; Figure 2C). Responses to 5FU-induced anemia also were tested at a myeloablative dose (150 mg/kg, IP). At days 5 through 15, Gata1-IE3.9int-DAPK2 mice again exhibited more severe anemia (P = .001 to .078 for mean hematocrits) as well as somewhat delayed recovery (see Figure S5D).
DAPK2 compromises erythropoiesis during anemia; exerts stage-specific proapoptotic effects; and is subject to EPO regulation in UT7epo erythroid progenitors. (A) The diagrammed construct was used to generate several independent transgenic DAPK2 founder lines. For 2 founder lines (F5 and F4), DAPK2 protein expression was assessed via Western blotting of primary splenocytes from phenylhydrazine-treated mice. (B) Erythropoiesis in Gata1-IE3.9int-DAPK2 mice is defective during phenylhydrazine induced anemia. Gata1-IE3.9int-DAPK2 mice were dosed with phenylhydrazine (52.5 mg/kg at 1 and 24 hours). Hematocrits (means ± SD) were then analyzed over a 12-day time-course. For a significant portion of Gata1-IE3.9int-DAPK2 mice, the anemia induced by phenylhydrazine was irreversible, and fatal (see Figure S5C). (C) Attenuated Epo-responsiveness in Gata1-IE3.9int-DAPK2 mice. Mice were dosed intraperitoneally with EPO (1000 U/kg). At the indicated intervals, hematocrits (means ± SD) then were determined. (D) Gata1-IE3.9int-DAPK2 erythroblast development is restricted at late developmental stages. Erythroid progenitor cells were expanded from Gata1-IE3.9int-DAPK2 and wild-type sibling bone marrow preparations. At day 3, frequencies of Kit+CD71highTER119−, Kit−CD71highTer119−, and Kit−CD71highTer119+ erythroblasts were determined. For Gata1-IE3.9int-DAPK2 erythroblasts, note the decreased frequencies of Kit−CD71highTer119+ erythroblasts. Numbers on plots are percentages of total gated cells. (E) For SP34-EX expansion cultures from Gata1-IE3.9int-DAPK2 and wild-type marrow preparations, frequencies of apoptotic cells also were assayed among developmentally staged erythroblasts (based on stage-specific cell surface markers and annexin-V staining). (F) siRNA knock-down of endogenous DAPK2 enhances UT7epo cell survival. Left panels outline the DAPK2-directed siRNA (and lentivirus vector) used, and the level of knock-down achieved (DAPK2 Western blot). The right panel illustrates increased survival of UT7epo cells due to DAPK2 siRNA. Here, cells were transduced, and isolated by FACS. Cells then were cultured in the presence of EPO (0.2 U/mL). At the hours indicated, viability was determined (via flow cytometric assay of PI positivity). Data are representative of 2 independent experiments. (G) EPO-regulation of DAPK2 kinase activity, and S318 phosphorylation. Possible effects of EPO on DAPK2 activity were studied in UT7epo cells stably transduced (at low MOI, and FACS-isolated) with a CGW-(flag)DAPK2 lentivirus. Here, UT7epo-(flag)DAPK2 cells were cultured for 16 hours in the absence of hematopoietic cytokines, and then exposed to EPO (5 U/mL) for the indicated intervals. The activity of immunoprecipitated (flag)DAPK2 then was assayed in vitro using MLC as a substrate (top panel). Assays of P-Ser19-MLC were by Western blotting (myosin light chain-2 p-Ser19 antibody; Cell Signaling Technology, Danvers, MA). Effects on DAPK2 phosphorylation at an inhibitory Ser318 site also were analyzed (bottom panel; DAPK2 p-Ser318 antibody; Santa Cruz Biotechnology, Santa Cruz, CA).
DAPK2 compromises erythropoiesis during anemia; exerts stage-specific proapoptotic effects; and is subject to EPO regulation in UT7epo erythroid progenitors. (A) The diagrammed construct was used to generate several independent transgenic DAPK2 founder lines. For 2 founder lines (F5 and F4), DAPK2 protein expression was assessed via Western blotting of primary splenocytes from phenylhydrazine-treated mice. (B) Erythropoiesis in Gata1-IE3.9int-DAPK2 mice is defective during phenylhydrazine induced anemia. Gata1-IE3.9int-DAPK2 mice were dosed with phenylhydrazine (52.5 mg/kg at 1 and 24 hours). Hematocrits (means ± SD) were then analyzed over a 12-day time-course. For a significant portion of Gata1-IE3.9int-DAPK2 mice, the anemia induced by phenylhydrazine was irreversible, and fatal (see Figure S5C). (C) Attenuated Epo-responsiveness in Gata1-IE3.9int-DAPK2 mice. Mice were dosed intraperitoneally with EPO (1000 U/kg). At the indicated intervals, hematocrits (means ± SD) then were determined. (D) Gata1-IE3.9int-DAPK2 erythroblast development is restricted at late developmental stages. Erythroid progenitor cells were expanded from Gata1-IE3.9int-DAPK2 and wild-type sibling bone marrow preparations. At day 3, frequencies of Kit+CD71highTER119−, Kit−CD71highTer119−, and Kit−CD71highTer119+ erythroblasts were determined. For Gata1-IE3.9int-DAPK2 erythroblasts, note the decreased frequencies of Kit−CD71highTer119+ erythroblasts. Numbers on plots are percentages of total gated cells. (E) For SP34-EX expansion cultures from Gata1-IE3.9int-DAPK2 and wild-type marrow preparations, frequencies of apoptotic cells also were assayed among developmentally staged erythroblasts (based on stage-specific cell surface markers and annexin-V staining). (F) siRNA knock-down of endogenous DAPK2 enhances UT7epo cell survival. Left panels outline the DAPK2-directed siRNA (and lentivirus vector) used, and the level of knock-down achieved (DAPK2 Western blot). The right panel illustrates increased survival of UT7epo cells due to DAPK2 siRNA. Here, cells were transduced, and isolated by FACS. Cells then were cultured in the presence of EPO (0.2 U/mL). At the hours indicated, viability was determined (via flow cytometric assay of PI positivity). Data are representative of 2 independent experiments. (G) EPO-regulation of DAPK2 kinase activity, and S318 phosphorylation. Possible effects of EPO on DAPK2 activity were studied in UT7epo cells stably transduced (at low MOI, and FACS-isolated) with a CGW-(flag)DAPK2 lentivirus. Here, UT7epo-(flag)DAPK2 cells were cultured for 16 hours in the absence of hematopoietic cytokines, and then exposed to EPO (5 U/mL) for the indicated intervals. The activity of immunoprecipitated (flag)DAPK2 then was assayed in vitro using MLC as a substrate (top panel). Assays of P-Ser19-MLC were by Western blotting (myosin light chain-2 p-Ser19 antibody; Cell Signaling Technology, Danvers, MA). Effects on DAPK2 phosphorylation at an inhibitory Ser318 site also were analyzed (bottom panel; DAPK2 p-Ser318 antibody; Santa Cruz Biotechnology, Santa Cruz, CA).
To examine possible stage-specific effects, an ex vivo expansion system was used to analyze bone marrow erythroblast development. Gata1-IE3.9int-DAPK2 Kit+CD71highTer119− (pro)erythroblasts formed at near wild-type frequencies (data not shown). Kit−CD71highTer119− progeny, however, were somewhat under-represented. At a Kit−CD71highTer119+ stage, this deficit was even more marked (Figure 2D). Results were reproducible, as also assessed during days 1 to 4 of culture (Figure S6D). Gata1-IE3.9int-DAPK2 erythroblast survival potentials also were studied. Here, Kit+CD71highTer119− erythroblasts were isolated at day 3, and replated in EPO at limiting doses. At 16 hours, annexin V+ cells were assayed. In Gata1-IE3.9int-DAPK2 erythroblasts, apoptosis occurred at increased frequencies, especially among Kit−CD71highTer119− cells (Figure 2E and Figure S6A-C).
In UT7epo cells, effects of siRNA-mediated DAPK2 knock-down on erythroid progenitor survival were studied using a DAPK2 siRNA lentivirus. UT7epo-siDAPK2 and control UT7epo-siLuc cells were prepared, and were plated in EPO at a limiting dose (0.2 U/mL). At the indicated intervals, frequencies of viable cells were determined. The knock-down of DAPK2 afforded substantial survival advantages (up to 42.8% over controls; Figure 2F). The observed comparably extended time-course for UT7epo cell death is attributed to the inclusion of FBS, and UT7epo's nature as an EPO-dependent yet permanent cell line.23-25
Possible EPO modulation of DAPK2 also was investigated. UT7epo-DAPK2 cells were cultured in the absence of cytokines, and EPO-exposed. (Flag)DAPK2 then was immunoprecipitated and assayed using an in vitro kinase assay. EPO-exposure stimulated DAPK2 activity approximately 200% (Figure 2G). DAPK2 Ser318 phosphorylation also was analyzed, and EPO was observed to also limit this inhibitory event (Figure 2G). EPO is known to stimulate several inhibitory factors in negative feedback routes including CIS,26 SOCS327 and a β-Trcp ubiquitin ligase.23 Apparent EPO stimulation of DAPK2 at least suggests a similar negative feedback role. Gata1-IE3.9int-DAPK2 (pro)erythroblasts, in addition, appeared to be attenuated in their transition from Kit−CD71highTer119− to Kit−CD71highTer119+ stages (see Figure 2D and Figure S6D). This finding raises the interesting additional possibility that DAPK2 also may exert regulatory effects on proerythroblast development.
Several findings indicate that DAPK2 may enforce significant negative regulation over (pro)erythroblast formation. (1) DAPK2's expression among hematopoietic cells is predominantly erythroid, and up-modulated during erythroblast development. (2) Gata1-IE3.9int-enforced DAPK2 expression compromises (pro)erythroblast survival and production, especially during anemia. This at least suggests anemia and/or stress-specific activation of DAPK2, and DAPKs interestingly can be up-modulated by hypoxia.28 (3) DAPK2's proapoptotic effects were exerted in a stage-selective fashion within EPO-dependent Kit−CD71highTer119− erythroblasts. (4) As analyzed in primary erythroblasts ex vivo, DAPK2 also may act to attenuate proerythro-blast development specifically at a Kit−CD71highTer119− to Kit−CD71highTer119+ transition. (5) In addition, DAPK2 may be EPO-modulated, and its knock-down enhances EPO-dependent erythroid progenitor cell survival. DAPK2 therefore comprises a new candidate attenuator of erythropoiesis which may network with EPO and BCL-XL/NIX pathways to control survival potentials, possibly via caspase-independent type II mechanisms.5-7,29 The nature of anemia- and/or stress-induced pathways that engage DAPK2 under these conditions, but not at steady state, also will be of significant interest to discover in future, and ongoing investigations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant R01HL44491. Core facility support also was provided via NIH P20 RR18789.
National Institutes of Health
Authorship
Contribution: J.F., D.Z., and M.M. performed the bulk of thiamphenicol-based splenocyte preparations and analyses, including transcriptome profiling (together with L.O. and D.W.); D.W. and E.H. worked with J.F. to prepare transgenic expression constructs and mice; E.H., D.W., and L.O. contributed to investigations using ex vivo expansions and flow cytometry; M.T. and B.T. contributed to the design, construction, and preparation of high titer lentiviral vectors. All authors contributed in meaningful ways to data interpretation, figure construction. and manuscript writing and preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Don M. Wojchowski, Director, Stem and Progenitor Cell Biology Program, Maine Medical Center Research Institute, 81 Research Drive, Scarborough, ME 04074; e-mail: wojchd@mmc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal