Abstract
Although CD38, a marker of poor prognosis in chronic lymphocytic leukemia (CLL), is known primarily as an ecto-enzyme, it has also been ascribed a receptor function. Interaction with its proposed ligand CD31 expressed on nurse-like cells would result in proliferative and survival-signals. Yet, in CLL, both homotypic and heterotypic CD31-CD38 interactions are expected to be rather ubiquitous. We analyzed whether CD38-CD31 interactions result in proliferative and antiapoptotic signals. We found a high expression of CD31 on CLL, irrespective of CD38 expression. Coculture of CD38high CLL with endothelial cells or CD31 transfected fibroblasts, with or without blocking CD31 or CD38 antibodies, did not result in increased survival or proliferation. Analysis of gene expression of most known regulators of apoptosis revealed no influence of coculture with CD31-expressing feeder cells. In conclusion, our data do not support an important contribution of CD38 triggering by CD31 to the proliferative and antiapoptotic state of the leukemic clone.
Introduction
A high expression level of CD38 on chronic lymphocytic leukemia (CLL) is associated with an unfavorable prognosis.1 This observation did not exceed the level of phenomenology until Deaglio et al recently attributed an important functional role to CD38 as a transmembrane receptor for the proposed ligand PECAM-1 (CD31, a member of the Ig superfamily).2 Because interaction of these molecules resulted in proliferative and survival signals in CLL cells,2 the aggressive clinical course in patients with CD38high CLL was suggested to result from the binding of the leukemic cells to CD31high nurse-like cells localized in the bone marrow. Moreover, in a recent article in Blood, the same group linked CD38 triggering to expression of ZAP70, another indicator of poor prognosis in CLL.3
However, besides on nurse-like cells, CD31 is highly expressed on multiple cell types, including endothelial cells and peripheral blood mononuclear cells (PBMCs) from healthy donors (reviewed in Newman4 ). Moreover, it has been reported that CLL cells also express variable levels of CD31.5 Therefore, in CLL, both homotypic and heterotypic CD31-CD38 interactions are expected to be rather ubiquitous.
We analyzed whether CD38-CD31 interactions among CLL cells and between CLL cells and endothelium result in proliferative and antiapoptotic signals, and whether the expression profile of apoptosis-regulating genes changes upon CD31-CD38 interaction.
Methods
Patient samples and cell lines
Approval for these studies was obtained from the Amsterdam Academic Medical Center Medical Ethical Committee. Informed consent was obtained in accordance with the Declaration of Helsinki. After written informed consent, CLL samples were obtained and handled as described before.6 After thawing, cells were cultured in Iscove modified Dulbecco medium (IMDM; Invitrogen, Carlsbad, CA), supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS; ICN Biomedicals, Meckenheim, Germany), 100 μg/mL gentamycin, and 5 mM l-glutamine (Invitrogen). All patient-derived PBMCs used in the experiments contained > 90% CD5+CD19+ cells. APC-labeled CD31-mAb (eBioscience, San Diego, CA) and PE-labeled CD38-mAb (IQ Products, Groningen, The Netherlands) were used to assess expression levels of these proteins.
ECRF24 cells, an immortalized CD31high human endothelial cell line,7 were kindly provided by R. D. Fontijn (Department of Medical Biochemistry, Academic Medical Center, Amsterdam, The Netherlands).
NIH-3T3 cells stably transfected with either a plasmid encoding human CD40L (3T40L) or negative control plasmid (3T3) were provided by D. van Baarle (Sanquin Blood Supply Foundation, Amsterdam, The Netherlands). Next, the 3T3 cell line was stably transfected with a human CD31-construct (3T31). This resulted in high expression levels of CD31 as measured by flow cytometry (FACScalibur; BD Biosciences, Breda, The Netherlands) and analyzed with CellQuest software (BD Biosciences).
Cell culture and measurement of apoptosis and proliferation
For coculture experiments, thawed CLL cells (106 cells/mL) were preincubated for 30 minutes with the blocking anti–CD31-mAbs HEC65 or HEC170 (Sanquin)8 or the anti-CD38-mAb AT1 (Santa Cruz Biotechnology, Santa Cruz, CA)9 at 10 μg/mL and subsequently cocultured with the ECRF24 cell line or irradiated (30 Gy) control, CD40-ligand- or CD31-transfected 3T3 feeder cells (plated at a 60% confluency).
For apoptosis measurements, cells were harvested at indicated time points, washed, labeled with FITC-labeled annexin V (IQ Products) and APC-labeled anti-CD19 antibodies (BD Biosciences) for 30 minutes, and analyzed by flow cytometry. Just before analysis, propidium iodide (PI) was added (final concentration 5 μg/mL). Annexin V–/PI−/CD19+ cells were designated viable.
For assessment of proliferation, freshly thawed PBMCs from CLL patients were labeled with carboxy fluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) and cocultured as described. At indicated time points, cells were harvested and proliferation was assessed by CFSE dilution (flow cytometry). As a positive control for proliferation, CLL cells were cocultured with 3T3 cells transfected with CD40-ligand in the presence of the oligo-dinucleotide CpG (ODN2006, Invitrogen).
RNA isolation and reverse transcription–multiplex ligation-dependent probe amplification assay
For the purpose of RNA isolation, 5 × 106 CLL cells from 6 patients were cocultured with irradiated control, CD40-ligand-, or CD31-transfected 3T3 feeder cells, and harvested after 3 days.
After total RNA was isolated using the GenElute mammalian total RNA miniprep kit (Sigma-Aldrich, St Louis, MO), a reverse transcription-multiplex ligation-dependent probe amplification assay (RT-MLPA) procedure was performed as described previously.10 For data presentation, gene expression levels were calculated relative to the average of 6 CLL samples cocultured with control 3T3 feeder cells. The resulting data were imported into the TIGR Multiexperiment viewer, version 4.1.11
Results and discussion
A high expression of CD31 was measured on CLL cells of both CD38high (n = 15; CD38+ cells > 30%) as well as CD38low patients (n = 8; CD38+ cells < 30%; Figure 1A).
Expression of CD31 on CLL or bystander cells does not influence survival and proliferation of CD38high CLL cells. (A) Like ECRF24 and CD31-transfected 3T3-cells (3T31), CLL cells have a high surface expression of CD31, irrespective of CD38 expression (MFI-R = ratio mean fluorescence APC-conjugated CD31/ MFI APC-conjugated isotype-control; CLL CD38low n = 8, CLL CD38high n = 15; error bars represent SEM; n.s. = not significant, unpaired 2-sided Student t test). (B) There is no significant difference in viability of CLL cells cocultured with 3T3 and 3T31 cells, with or without addition of blocking CD31 antibodies (HEC65 and HEC170). CLL cells cocultured with 3T3 transfected with CD40-ligand (3T40L) have a significantly increased survival (viability shown at 5 days; * P < .05, paired 2-sided Student t test). (C) There is no significant modulation of viability of CLL-cells cocultured with 3T3 and 3T31 cells, with or without addition of the anti-CD38 antibody AT1 (viability shown at 5 days). (D) Combined CD40 and TLR-9 triggering induces marked proliferation of CLL cells.
Expression of CD31 on CLL or bystander cells does not influence survival and proliferation of CD38high CLL cells. (A) Like ECRF24 and CD31-transfected 3T3-cells (3T31), CLL cells have a high surface expression of CD31, irrespective of CD38 expression (MFI-R = ratio mean fluorescence APC-conjugated CD31/ MFI APC-conjugated isotype-control; CLL CD38low n = 8, CLL CD38high n = 15; error bars represent SEM; n.s. = not significant, unpaired 2-sided Student t test). (B) There is no significant difference in viability of CLL cells cocultured with 3T3 and 3T31 cells, with or without addition of blocking CD31 antibodies (HEC65 and HEC170). CLL cells cocultured with 3T3 transfected with CD40-ligand (3T40L) have a significantly increased survival (viability shown at 5 days; * P < .05, paired 2-sided Student t test). (C) There is no significant modulation of viability of CLL-cells cocultured with 3T3 and 3T31 cells, with or without addition of the anti-CD38 antibody AT1 (viability shown at 5 days). (D) Combined CD40 and TLR-9 triggering induces marked proliferation of CLL cells.
Culture of CLL cells of 4 CD38high patients at high (5 × 106/mL) and low (0.5 × 106/mL) density for 5 days, with or without addition of blocking anti-CD31 antibodies, did not result in modulation of apoptosis or proliferation (data not shown).
To analyze heterotypic interactions between CLL cells and endothelium, CLL cells of 6 CD38high patients were cocultured with the CD31high endothelial cell line ECRF24. CD38low cells and addition of the blocking anti-CD31 antibodies were used in control experiments. No significant differences in apoptosis or proliferation were observed between any of the conditions after 24, 48, and 72 hours (data not shown).
Because proliferation of ECRF24 cells could not be inhibited by irradiation without concomitant induction of cell death, for prolonged coculture experiments we used CD31-transfected mouse fibroblasts. CLL cells of 10 CD38high and 5 CD38low patients were cocultured with either 3T3 or 3T31 cells, with or without the addition of blocking CD31 antibodies. Coculture for up to 7 days did not result in proliferation in any of the tested conditions (data not shown). In addition, no modulation of apoptosis could be observed. In sharp contrast, coculture of these CLL cells with 3T40L-transfected fibroblasts rescued cells from apoptosis (Figure 1B). When these experiments were repeated in the presence of the AT1 antibody to block CD38, neither proliferation nor modification of apoptosis were seen (Figure 1C).
Besides the antiapoptotic effects of CD40L stimulation, coculture of CLL with 3T40L cells in the presence of the oligo-dinucleotide CpG induced marked proliferation (Figure 1D), demonstrating that receptor triggering (CD40, Toll-like receptor-9) can indeed influence survival and proliferation of CLL cells.
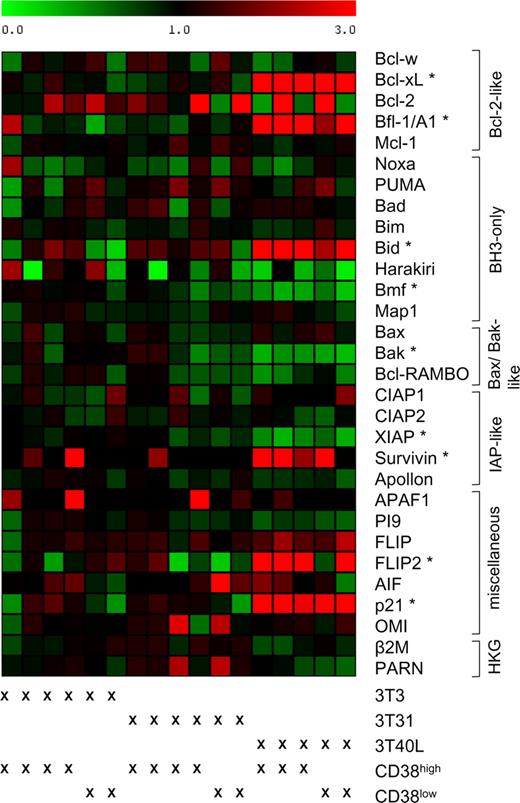
In addition to these functional experiments, we analyzed whether coculture of CD38high CLL cells with CD31 expressing feeder cells induced changes in the expression-profile of apoptosis regulating genes. CD31 interaction for up to 120 hours did not result in significant differences in the expression of apoptosis regulators (Figure 2). In contrast, coculture with CD40 ligand-expressing feeder cells induced characteristic changes in this gene expression profile (among which are up-regulation of BCL-XL, bfl-1/A1, and Bid). Of note, survivin, which in most cells is expressed exclusively during the G2/M cell-cycle phase,12 was not up-regulated upon CD31 stimulation, whereas CD40-ligand stimulation clearly induced its expression. This might be seen as another argument against an important role of CD31-CD38 interactions in the proliferation of CLL cells.
Coculture of CLL cells with CD31-transfected fibroblasts does not result in an altered expression profile of apoptosis-regulating genes. CLL samples were cocultured for 3 days with 3T3, 3T31, or 3T40L cells (CD38low n = 2; CD38high n = 4 for 3T3; 3T31 and n = 3 for 3T40L). The relative expression level of indicated genes, as assessed by RT-MLPA, was related to the average expression in the CLL samples cocultured with 3T3 cells. The resulting matrix was imported into the program MultiExperiment Viewer, and values were assigned green or red colors: green for values between 0 and 1, indicating down-regulation, and red for values greater than 1, indicating up-regulation. The CLL samples are ordered as indicated below the matrix. In the right column, the genes are ordered by functional category (HKG = housekeeping genes; β2M = β-2-microglobulin). A significant change in expression level was defined as a 2-fold up- or down-regulation of the average expression of a gene in samples cocultured with 3T31 or 3T40L cells compared with samples cocultured with 3T3 cells and a statistical significant difference with a P value less than .05 (2-sided Student t test for 5 paired samples). No significant differences were found after coculture with 3T3 or 3T31 cells; genes with a significant differential expression level after coculture with 3T40L are denoted with *. To exclude the possibility that variations in gene expression occur at time points earlier or later than 72 hours, additional RNA of 2 CD38high CLL patients was isolated after 24 and 120 hours of coculture. At these additional time points, no differences were found (data not shown).
Coculture of CLL cells with CD31-transfected fibroblasts does not result in an altered expression profile of apoptosis-regulating genes. CLL samples were cocultured for 3 days with 3T3, 3T31, or 3T40L cells (CD38low n = 2; CD38high n = 4 for 3T3; 3T31 and n = 3 for 3T40L). The relative expression level of indicated genes, as assessed by RT-MLPA, was related to the average expression in the CLL samples cocultured with 3T3 cells. The resulting matrix was imported into the program MultiExperiment Viewer, and values were assigned green or red colors: green for values between 0 and 1, indicating down-regulation, and red for values greater than 1, indicating up-regulation. The CLL samples are ordered as indicated below the matrix. In the right column, the genes are ordered by functional category (HKG = housekeeping genes; β2M = β-2-microglobulin). A significant change in expression level was defined as a 2-fold up- or down-regulation of the average expression of a gene in samples cocultured with 3T31 or 3T40L cells compared with samples cocultured with 3T3 cells and a statistical significant difference with a P value less than .05 (2-sided Student t test for 5 paired samples). No significant differences were found after coculture with 3T3 or 3T31 cells; genes with a significant differential expression level after coculture with 3T40L are denoted with *. To exclude the possibility that variations in gene expression occur at time points earlier or later than 72 hours, additional RNA of 2 CD38high CLL patients was isolated after 24 and 120 hours of coculture. At these additional time points, no differences were found (data not shown).
These data are to some extent discordant with the findings of Deaglio et al.2 This could result from differences in experimental setup, such as cell purity and the use of interleukin-2, which may have direct effects on CLL cells, or differences in readouts used for assessment of apoptosis and proliferation and the cell population analyzed.
Although Damle et al recently showed a strong correlation between expression of CD38 and KI-67, a marker of cell-cycle entry,13 our data do not support an important contribution of CD38 triggering by CD31 to the proliferative, antiapoptotic state of the leukemic clone. It may be that in CLL CD38 functions primarily as an ecto-enzyme in accordance with its role on various cell types of the immune system (reviewed in Lund et al14 ).
Thus, the functional significance of CD38 expression on CLL remains to be determined.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was partially funded by a personal grant from the Dutch Cancer Society (UVA 2006-3712 to S.H.T.). A.P.K. is supported by a Veni grant from The Netherlands Organization for Health Research and Development (ZonMw).
Authorship
Contribution: S.H.T. designed research, performed experiments, analyzed data, and wrote the paper; R.S. and D.M.L. performed experiments; M.H.v.O. reviewed the manuscript; and A.P.K. designed research and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sanne H. Tonino, Department of Hematology, Academic Medical Center, K0-152, PO Box 22660, 1100 AZ Amsterdam, The Netherlands; e-mail: s.h.tonino@amc.uva.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal