Abstract
Inhibitory killer cell immunoglobulin receptors (KIR) bind to major histocompatibility complex antigens. Concise knowledge of KIR ligands allows prediction of natural killer (NK)–cell alloreactivity after hematopoietic stem cell transplantation. KIR3DL1 binds to the Bw4 epitope on HLA-B antigens. Although the same epitope is also found on 4 HLA-A antigens (HLA-A23/24/25/32), these are not currently regarded as KIR3DL1 ligands. We show that expression of HLA A*2301, A*2402, or A*3201 but not HLA A*2501 protects target cells from lysis by KIR3DL1+ NK cells. KIR3DL1+ NK cells from donors expressing the Bw4 epitope on an HLA-A antigen only are fully functional and capable of lysing Bw4− target cells. HLA A25 differs at amino acid 90, close to the serologic Bw4 epitope, from A23/24/32 and from Bw4+ HLA-B antigens. These data suggest that HLA-A antigens should be taken into consideration when assessing the potential for NK alloreactivity after hematopoietic stem cell transplantation.
Introduction
Natural killer (NK) cells achieve tolerance through inhibitory receptors for major histocompatibility complex (MHC) antigens.1 In humans, the 2 main classes of inhibitory MHC receptors are (1) the killer cell immunoglobulin-like receptors (KIRs) with distinct specificity for supertypic epitopes shared by groups of HLA class I antigens,2 and (2) the NKG2A receptor, which binds to HLA-E and therefore has broad specificity.3 Full understanding of the KIR binding patterns is essential for predicting NK-cell alloreactivity in HLA-mismatched,4,5 and possibly also HLA-identical6 hematopoietic stem cell transplantation. There is consensus that KIR2DL1 binds to HLA-C antigens expressing lysine at position 80; KIR2DL2 and KIR2DL3 are engaged by HLA-C antigens with asparagine at position 80; KIR3DL1 binds to HLA-B antigens with the Bw4 serologic specificity derived from leucine/arginine at positions 82/83. Initial data on KIR3DL1 indicated binding of Bw4 epitopes on both HLA-B and HLA-A,7 whereas a later study using antibody blocking experiments suggested that only HLA-B antigens with Bw4 specificity acted as ligands for KIR3DL1.8 Consequently, HLA-A antigens with Bw4 specificity have been ignored when assessing KIR ligand mismatches in hematopoietic transplantation.9 We show that the HLA-A*2301, A*2402, and A*3201 but not A*2501 are ligands for KIR3DL1 and should be taken into consideration when assessing the potential for NK alloreactivity in hematopoietic stem cell transplantation.
Methods
Preparation of NK clones
NK cells of 2 healthy donors were isolated from whole blood using the RosetteSep-method (StemCell Technologies, Vancouver, BC). Both donors lacked the gene for KIR3DS1, implying that the antibody used to detect KIR3DL1 (Z27) bound to the inhibitory isoform only. Allele-level KIR3DL1 genotyping showed that both donors possessed 2 KIR3DL1 alleles (donor 1, KIR3DL1*001 and KIR3DL1*002; donor 2, KIR3DL1*001 and KIR3DL1*005). Whereas donor 1 expressed the Bw4 epitope only on one HLA-A but not on any HLA-B antigens (HLA class I typing: A1/32, B8/60, C3/7, Bw4-antigen bold), donor 2 expressed the Bw4 epitope on one HLA-B antigen, but not on HLA-A antigens (HLA class I typing: A1/29, B8/49, C7/16).
NK clones positive for KIR3DL1 and negative for other KIR and NKG2A were obtained by limiting dilution culture of NK cells depleted for KIR2DL/S1/2/3 and NKG2A and then positively selected for KIR3DL1 using phycoerythrin-conjugated antibodies (clones EB6b (KIR2DL/S1), GL183 (KIR2DL/S2/3), Z27 (KIR3DL/S1), and Z199 (NKG2A), all from Coulter Immunotech, Marseille, France). Limiting dilution cloning of NK cells was performed as described.4 Emerging clones were controlled for expression of KIR3DL1, absence of KIR2DL/S1/2/3 (Figure 1B), and absence of KIR3DL2 (clone Q66) and ILT2 (clone HP-F1) surface expression before being used in functional experiments.
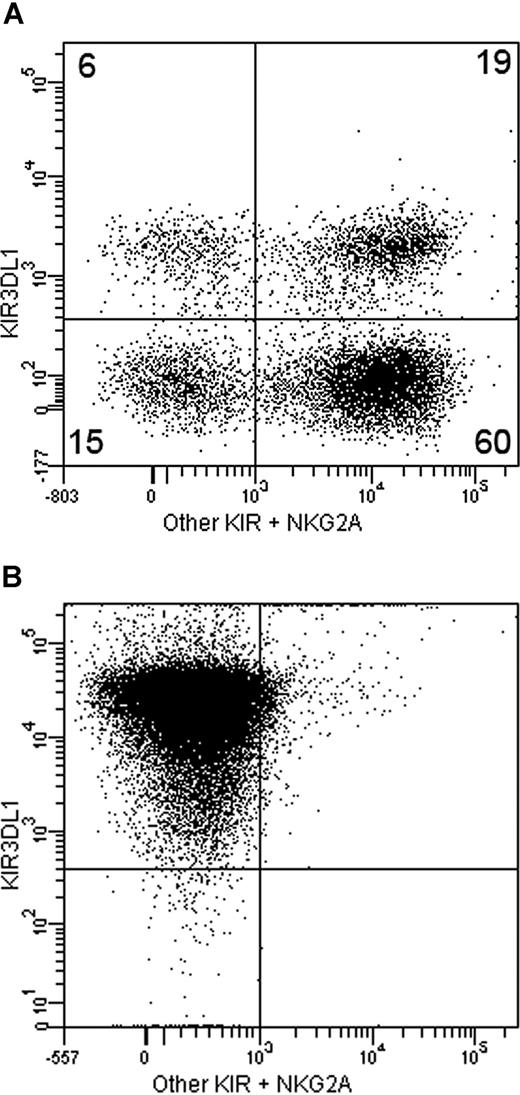
Inhibitory MCH receptor repertoire of selected and unselected NK cells. (A) Inhibitory MHC receptor repertoire of donor 1 (expressing Bw4 on HLA-A but not HLA-B), showing 6% of NK cells expressing KIR3DL1 as their sole inhibitory MHC receptor. Numbers on plot are the percentages of total cells in the gate. (B) The same analysis performed on clonally expanded NK cells after depletion for KIR2DL/S1/2/3 and NKG2A and positive selection for KIR3DL1.
Inhibitory MCH receptor repertoire of selected and unselected NK cells. (A) Inhibitory MHC receptor repertoire of donor 1 (expressing Bw4 on HLA-A but not HLA-B), showing 6% of NK cells expressing KIR3DL1 as their sole inhibitory MHC receptor. Numbers on plot are the percentages of total cells in the gate. (B) The same analysis performed on clonally expanded NK cells after depletion for KIR2DL/S1/2/3 and NKG2A and positive selection for KIR3DL1.
NK clones from an HLA-A-25–expressing patient transplanted from an HLA-A-32–expressing (otherwise KIR-ligand matched) donor were produced from unselected NK cells after transplant and tested for alloreactivity against previously cryopreserved patient cells as part of the standard evaluation of patients receiving haploidentical hematopoietic stem cell transplantation at our center. Approval was obtained from the Umbria Region Ethics Committee and from the Perugia University institutional review board and written informed consent was obtained in accordance with the Declaration of Helsinki.
Chromium release assays
NK-cell clones were used as effectors in standard 51Cr release cytotoxicity assays using as targets allogeneic or autologous phytohemagglutinin lymphoblasts or Epstein-Barr virus–transformed B-cell lines. Interaction of KIR3DL1 and its MHC target antigens was disrupted by adding saturating concentrations of antibodies directed against either KIR3DL1 (clone Z27, IgG1) or class I MHC (clone A6-136, IgM). Coincubation with an antibody directed against the nonexpressed KIR2DL1 served as a negative control (clone 11PB6, IgG1; all antibodies were kind gifts of A. Moretta, Universitá degli Studi di Genova, Genova, Italy).
Target cells
Target cell lines used included Epstein-Barr virus B-cell and autologous or allogeneic polyclonal T-cell lines. Cell lines negative for Bw4 (COX: HLA-A1/−, HLA-B8/−, HLA-Cw7/−) or expressing Bw4 on an HLA-B antigen but not on HLA-A (LADA: HLA-A2/A80, HLA-B*5703/B7, HLA-Cw7/−) were used as controls. Cell lines expressing Bw4 on HLA-A antigens only expressed the following antigens: HLA-A*2301/−, HLA-B14/−, HLA-Cw8/− (WT51); HLA-A*2402/−, HLA-B35/−, HLA-Cw4/− (TISI); HLA-A*2402/A3, HLA-B7/B40, HLA-Cw2/Cw7 (W00); HLA-A*2501/30, B18/B42, HLA-Cw*12/Cw*17 (A25-1); HLA-A*2501/1, HLA-B8/B18, Cw7/− (A25-2); HLA-A3/A*3201, HLA-B35/B64, HLA-Cw4/Cw8 (PUTFRA); HLA-A*3201/A1, HLA-B8/B60, HLA-Cw3/Cw7 (A32-1).
Results
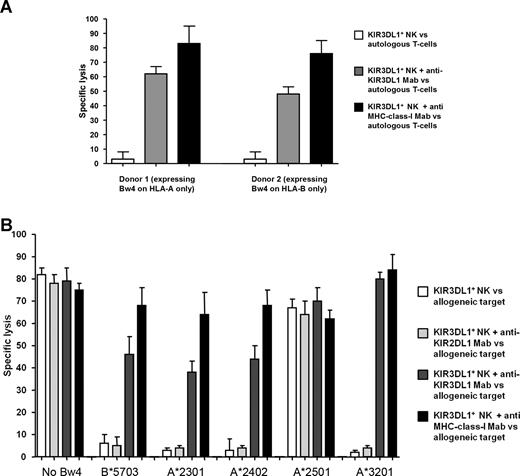
NK cells expressing KIR3DL1 as their sole inhibitory HLA-receptor were found at similar frequencies in donor 1 who expressed the Bw4 epitope on only an HLA-A antigen (6%, Figure 1A) and donor 2 who expressed it on only an HLA-B antigen (5%). NK clones expressing KIR3DL1 but no other inhibitory HLA receptor were generated from both donors (Figure 1B). Tolerance of KIR3DL1 positive clones to autologous phytohemagglutinin T-cell lymphoblasts was broken by disrupting the interaction of KIR3DL1 and target HLA-antigens by an antibody directed against KIR3DL1 or an HLA class I antibody (Figure 2A).
Cytotoxicity of KIR3DL1+ NK clones against target cells with or without expression of Bw4. (A) KIR3DL1+ NK clones of both donor 1 expressing Bw4 on HLA-A only and donor 2 expressing Bw4 on HLA-B only are tolerant to autologous target cells. Lysis can be reconstituted in both cases by antibody-blocking of either KIR3DL1 on NK cells or MHC class I on target cells. (B) KIR3DL1+ NK clones lyse target cells lacking Bw4 or expressing A*2501, whereas target cells expressing A*2301, A*2402, or A*3201 are protected from lysis, similar to target cells expressing an HLA-B antigen containing the Bw4 epitope (B*5703). Lysis of target cells expressing A*2301/A*2402/A*3201 or B*5703 is reconstituted by antibodies directed against KIR3DL1 or MHC class I but not by control antibody (KIR2DL1). Results of experiments with different target cells expressing the same allele were pooled.
Cytotoxicity of KIR3DL1+ NK clones against target cells with or without expression of Bw4. (A) KIR3DL1+ NK clones of both donor 1 expressing Bw4 on HLA-A only and donor 2 expressing Bw4 on HLA-B only are tolerant to autologous target cells. Lysis can be reconstituted in both cases by antibody-blocking of either KIR3DL1 on NK cells or MHC class I on target cells. (B) KIR3DL1+ NK clones lyse target cells lacking Bw4 or expressing A*2501, whereas target cells expressing A*2301, A*2402, or A*3201 are protected from lysis, similar to target cells expressing an HLA-B antigen containing the Bw4 epitope (B*5703). Lysis of target cells expressing A*2301/A*2402/A*3201 or B*5703 is reconstituted by antibodies directed against KIR3DL1 or MHC class I but not by control antibody (KIR2DL1). Results of experiments with different target cells expressing the same allele were pooled.
NK clones expressing KIR3DL1 as their only inhibitory HLA receptor readily lysed target cells that did not possess HLA antigens expressing the Bw4 epitope, and addition of blocking antibodies directed against KIR or HLA did not increase lysis of target cells (Figure 2B group “No Bw4”). In contrast, NK-cell clones expressing only KIR3DL1 did not lyse cells expressing an HLA-B antigen with Bw4 specificity (such as HLA-B*5703). Lysis was reconstituted by adding blocking antibodies directed against either KIR3DL1 or HLA class I. Lysis was not reconstituted by control antibodies directed against other KIRs, such as KIR2DL1 (Figure 2B group B*5703). Similarly, NK clones expressing KIR3DL1 did not lyse target cells expressing HLA-A*2301, A*2402, or A*3201, and lysis was reconstituted by blocking antibodies directed against KIR3DL1 or HLA class I (Figure 2B groups A*2301, A*2402, and A*3201). In contrast, clones expressing KIR3DL1 lysed target cells expressing HLA-A*2501, and lysis was not increased by anti-KIR3DL1 or anti-HLA antibodies. These data indicate that HLA-A*2501 is not a ligand for KIR3DL1.
We had the opportunity to study an acute myeloid leukemia patient expressing the HLA-A*2501 antigen who had received a hematopoietic cell transplant from a haploidentical brother expressing HLA-A*3201. Donor and patient were matched for other KIR ligands. Functional analyses of NK clones of donor origin performed 14 weeks after transplantation revealed that 3 of 43 NK clones lysed patient-cryopreserved cells. Phenotypical analysis of these clones showed expression of KIR3DL1 and absence of other inhibitory HLA receptors. These data demonstrate the existence and function of an HLA-A KIR3DL1-ligand mismatch in graft-versus-host direction.
Discussion
We have shown that the HLA-A antigens A*2301, A*2402, and A*3201 but not A*2501 are ligands of KIR3DL1. Our results confirm and extend recently published data on binding of HLA-A24 tetramers to KIR3DL1.10 They do not concur with early findings that an anti-KIR3DL1 antibody did not reconstitute lysis of 721.221 cells transfected with HLA-A*2403 or A*2501.8 In light of the present findings, it is reasonable to deduce that the KIR3DL1-positive clones analyzed in this early report coexpressed NKG2A, which protected HLA-A but not HLA-B transfectants, because the HLA-A alleles but not the HLA-B alleles used in that study contain leader sequences that promote HLA-E expression.11 Notably, in the present study, NK clones not expressing NKG2A were used for reconstitution experiments. An alternative explanation is that allelic diversity within HLA-antigens (ie, differences between HLA-A*2402 and HLA*A2403) affects binding by KIR3DL1.
The clinical significance of our findings is underscored by observations in a haploidentical stem cell transplant recipient. The KIR3DL1-ligand mismatch on HLA-A allowed for NK alloreactivity.
In conclusion, the present findings show that HLA-A antigens A*2301, A*2402, and A*3201 should be taken into account when assessing potential NK alloreactivity in donor-recipient pairs who are candidates for hematopoietic stem cell transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
M.S. is supported by research grants from the Swiss Cancer League (grant BIL OCS 01 597-08-2004) and the Swiss National Science Foundation (grant PBBSB-107328).
Authorship
Contribution: M.S. designed research, collected data, and drafted the manuscript; L.R. and A.M. performed research and analyzed and interpreted data; M.C. performed research; and A.V. designed research, analyzed and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin Stern, Department of Hematology, University Hospital, Petersgraben 4, 4031 Basel, Switzerland; e-mail: sternm@uhbs.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal