Abstract
CD137 (4-1BB) is a costimulatory mol-ecule that can be manipulated for the treatment of cancer and autoimmune disease. Although it is known that agonistic antibodies (mAbs) against CD137 enhance the rejection of murine tumors in a natural killer (NK) cell– and T cell–dependent fashion, the mechanism for NK dependence is poorly understood. In this study, we evaluated the ability of 2 different glycoforms of a chimerized antihuman CD137 mAb, an aglycosylated (GA) and a low fucose form (GG), to react with human NK cells. Both mAbs bound similarly to CD137 and partially blocked the interaction between CD137 and CD137 ligand. However, unlike GA mAb, immobilized GG mAb activated NK cells and enhanced CD137 expression. These effects were seemingly dependent on Fc interaction with putative Fc receptors on the NK-cell surface, as only the immobilized Fc-fragment of GG was required for CD137 expression. Furthermore, CD137 expression could be enhanced with antibodies directed against non-CD137 epitopes, and the expression levels directly correlated with patterns of Fc-glycosylation recognized to improve Fc interaction with Fcγ receptors. Our data suggest that CD137 can be enhanced on NK cells in an Fc-dependent fashion and that expression correlates with phenotypic and functional parameters of activation.
Introduction
CD137 (4-1BB), a member of the TNF receptor superfamily, is increasingly recognized as an important target for the treatment of both cancer and autoimmunity.1-5 Specifically, in murine models, it is clear that ligation of CD137 on the surface of activated T cells, through either CD137 ligand (CD137L) or agonistic monoclonal antibodies (mAbs), potentiates the immune response to weakly immunogenic tumors in a natural killer (NK)–dependent fashion.3,6 Unlike other costimulatory-based antitumor immunotherapies (eg, CTLA-4 blockade), CD137 ligation does not induce self-reactivity, but rather has therapeutic benefit in multiple murine models of autoimmune disease such as rheumatoid arthritis,7 systemic lupus erythematosus,8 and inflammatory bowel disease.9 In many studies, functional conclusions regarding CD137 have been based on mAb or fusion protein manipulation of receptor/ligand pathways, with the assumption being that observed effects were secondary to Fab region or ligand interaction with CD137. Importantly, little attention has been paid to the potential link between the Fc region of these molecules and alternate pathways of CD137 regulation through Fc receptors (FcγRs)
It is now evident that Fc cross-linking of FcγRIII (CD16) on human NK cells induces cellular activation defined by both phenotypic change and release of proinflammatory cytokines.10 The affinity and functional consequences of the interaction between Fc and FcγRIII is dependent on the presence of oligosaccharides (N-glycan) covalently attached at asparagine 297 (Asn297) of the Fc heavy chain.11 For example, Fc fragments with low fucose content at Asn297 have enhanced binding affinity for FcγRIII.12-14 In addition, aglycosylated Fc fragments are unable to efficiently bind the FcγRIII.15,16 The interaction between Fc-FcγRs also has clinical implications, as it is now evident that polymorphisms within the FcγRIII (eg, V/F at amino acid position 158), which impact Fc-FcγRIII interactions, can be used to define the therapeutic efficacy of targeted anticancer therapeutics such as rituximab.17,18
Based on the therapeutic potential of anti-CD137 mAbs and the recognized importance of Fc-FcγR interactions on mAb function, in collaboration with GTC Biotherapeutics, 2 chimeric anti-CD137 mAbs were developed. The first, a glycosylated form (GG) is likely to cross-link the FcγR and the second, an aglycosylated form (GA), is unlikely to efficiently cross-link the FcγR. Because of the recognized role of NK cells in the antitumor function of anti-CD137 mAbs in murine models, we initially hypothesized that interleukin-2 (IL-2)–stimulated human NK cells would express CD137 and that ligation with chimeric anti-CD137 mAb would result in cytokine release and degranulation.
Surprisingly, we observed that, after IL-2 stimulation and subsequent culture, human NK cells do not express high levels of CD137. However, CD137 is enhanced on IL-2–stimulated human NK cells after culture in the presence of immobilized glycosylated mAbs or papain-cleaved Fc fragments. The ability to enhance CD137 expression is independent of the antigen specificity of the Fab region, and the magnitude of CD137 expression is associated with patterns of glycosylation known to enhance the interaction between Fc and FcγRs. These data suggest that “agonistic effects” of select antibodies on costimulatory molecules may be in part secondary to Fc-FcγR interactions and provide important insight into the design of antibodies intended to manipulate costimulatory pathways for clinical benefit.
Methods
All experimental work related to human materials was approved by the University of Maryland's institutional review board (IRB), under IRB number H-27785.
Chimeric monoclonal antibodies
Mouse anti–human CD137 mAb (G6) was generated in the laboratory of L. C. A glycosylated chimeric version of G6, hereafter called GG, was developed by replacing the mouse IgG2a Fc region with a human IgG1 Fc region. Likewise, an aglycosylated (GA) chimeric anti-CD137 mAb was created by mutating Asn to glutamine (Gln) at amino acid position 297 in the Fc region. Both GA and GG mAbs were produced in the milk of transgenic goats. Cetuximab (hereafter called CTM) was obtained through the Greenebaum Cancer Center (University of Maryland Medical System, Baltimore). For flow cytometric experiments, all chimeric mAbs were directly labeled to allophycocyanin (APC) through the custom conjugation service of Invitrogen (Invitrogen, Carlsbad, CA).
Cells and transfectants
Human blood samples were purchased (Biological Specialty Corporation, Colmar, PA) and whole peripheral blood mononuclear cells (PBMCs) were isolated using density-gradient centrifugation. Freshly purified resting NK cells (CD3−CD56+CD16+ as defined by flow cytometry) were obtained by negative magnetic cell sorting using NK-cell isolation beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. Chinese hamster ovary (CHO) cells stably transfected with human CD137 receptor and nontransfected wild-type controls were established in the laboratory of L.C. CHO cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biological, Lawrenceville, GA), 1% penicillin/streptomycin (P/S), 1% Glutamax-1 (both Invitrogen), and 1% HEPES (Mediatech, Herndon, VA). K562 cells were cultured in RPMI 1640 supplemented with 10% FBS, 1% P/S, and 1% Glutamax-1.
In vitro NK experiments
Fresh purified resting NK cells (CD3−CD56+CD16+ as defined by flow cytometry; > 90% purity) were stimulated for 72 hours in RPMI 1640 supplemented with 10% FBS, 1% P/S, 1% HEPES, 1% Glutamax-1, and 200 IU/mL recombinant human IL-2 (rhIL-2, aldesleukin; Chiron, Emeryville, CA). After 72 hours, NK cells were washed 3 times with phosphate-buffered saline and resuspended in RPMI 1640 supplemented with 10% FBS, 1% P/S, 1% HEPES, and 1% Glutamax-1. IL-2–stimulated NK cells were plated at a concentration of 2 × 105 cells/well in a 96-well flat-bottom plate in the presence of either precoated (immobilized) or soluble chimeric mAb or polyclonal human IgG1 (huIgG1; Sigma-Aldrich, St Louis, MO) at a concentration of 20 μg/mL. Control wells were precoated overnight with RPMI 1640 supplemented with 10% FBS to minimize cross-linking of soluble positively charged immunoglobulins present in the culture media. At the indicated time points, NK cells and cell-free supernatants were harvested and analyzed for cell surface expression of defined proteins and secreted cytokines.
Flow cytometry
NK-cell phenotype was determined by staining with directly conjugated mouse antihuman mAbs against CD3, CD56, CD16, CD69, CD54, and CD137 (BD Biosciences, San Diego, CA). Unless indicated otherwise, CD137 expression was measured using commercial mouse anti–human CD137 mAb (clone 4B4-1). In some experiments, NK cells were stained with annexinV/7-AAD (annexin V apoptosis detection kit I; BD Biosciences) to distinguish live cells from apoptotic and/or necrotic cells. The number of degranulating NK cells was determined by staining with CD107a mAb (BD Biosciences). In brief, NK-cell cultures were incubated for 1 hour at 37°C in 5% CO2 after which brefeldin A and monensin (both BD Biosciences) were added. NK cells were cultured for an additional 5 hours and subsequently stained for CD56 and CD16. Directly conjugated mouse IgGs were used as isotype controls. A minimum of 10 000 events were acquired using a BD LSR II flow cytometer and analyzed with BD FACS DIVA Software (BD Biosciences).
Analysis of collected cell-free supernatants
Cell-free culture supernatants were collected at indicated time points during incubation. Concentrations of IFN-γ and TNF-α were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (BD Biosciences).
Flow cytometry–based competitive binding assays
CD137-expressing CHO cells were incubated with different concentrations (0.8 μg/mL-100 μg/mL) of G6, GA, and GG mAb at 4°C for 1 hour. Purified mouse IgG2a (muIgG2a) and huIgG1 (BD Biosciences) were used as control antibodies at a concentration of 100 μg/mL. Next, cells were incubated with 5 μg/mL human CD137L-muCD8 (Ancell, Bayport, MN) for 45 minutes at 4°C. Cells were further stained with PE-conjugated anti-muCD8 mAb (BD Biosciences) and acquired using a BD LSR II flow cytometer.
Generation of Fab fragments and Fc fragments
Fab and Fc fragments from GG, CTM, and huIgG1 were generated by papain digestion followed by protein A and protein L chromatography using an ImmunoPure Fab Preparation kit (Pierce, Rockford, IL) according to the manufacturer's instructions. Briefly, all mAbs were digested into Fc and Fab fragments using papain. The digests were dialyzed against protein A or protein L binding solutions and subsequently passed through protein A or protein L columns, and the flow through, containing Fab or the Fc fragments, respectively, was collected. Purity, determined by both immunoblotting and ELISA, was greater than 75% and 95% for the Fc and Fab fractions, respectively.
Compositional monosaccharide analysis of IgG1-Fc
Purified Fc fragments (100-200 μg) were diluted to 200 μL using deionized water and mixed with an equal volume of 4N TFA (trifluoroacetic acid; Sigma-Aldrich). Samples were hydrolyzed at 100°C for 5 hours. After lyophilization, the residues were reconstituted with water prior to high-performance anion-exchange chromatography (HPAEC) analysis. The chromatography was performed on a Dionex DX600 chromatography system (Dionex, Sunnyvale, CA) equipped with an electrochemical detector (ED50). Monosaccharides were separated on a CarboPac PA10 (Dionex) column (4 × 250 mm) with an isocratic 18 mM NaOH as the eluent. Monosaccharides were detected by the pulsed amperometric electrochemical detector with the following waveforms (potentials and durations): E1 = +0.05 V (T1 = 0 to 0.4 seconds), E2 = +0.75 V (T2 = 0.41 to 0.6 seconds), and E3 = −0.15 V (T3 = 0.61 to 1 seconds). Typically, 10 μL of sample containing 0.1 to 1 nmol of monosaccharides was injected. For quantification, the peaks corresponding to the monosaccharides were integrated and peak areas were normalized to molar quantity by comparison with standard solution of monosaccharide standards.
Cytotoxicity assays
NK cytotoxicity was measured by 4-hour chromium-51 (51Cr)–release assays. K562 tumor targets were labeled with 150 μCi (5.55 MBq) 51Cr (Na251CrO4; Perkin Elmer, Shelton, CT) for 1 hour at 37°C in 5% CO2. After incubation, cells were washed 2 times and incubated for an additional 30 minutes to reduce spontaneous release. Finally, tumor targets were plated at 5000 targets/well in triplicate wells in a 96-well V-bottom plate. Effector cells consisted of CD3−CD56+ NK cells that were IL-2 stimulated for 72 hours and subsequently cultured for 24 hours in the presence of immobilized GA or GG mAb. NK effectors (100 μL) were added at indicated effector-target ratios to give a final volume of 200 μL per well. After 4 hours of incubation, 100 μL supernatant was harvested and mixed with 100 μL scintillation cocktail (Perkin Elmer) in a 96-well sample plate (Perkin Elmer). Its radioactive content was measured using a gamma-counter and the percentage of specific lysis was calculated using the formula: 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
Statistical analysis
A Q-Q plot was used to assess whether normality could be assumed for the flow cytometric parameters. Statistical significance, as measured by a 2-sided paired Student t test (or the nonparametric sign test if the normality was not plausible) was calculated using Excel v2003 (Microsoft, Redmond, WA) and Splus (Insightful, Seattle, WA), based on the number of experiments as indicated in the figure legends. NK-cell cultures with immobilized GA mAb were used as control cultures to calculate statistical differences. Differences were considered to be significant at P value less than .05.
Results
Immobilized glycosylated mAbs enhance CD137 expression on human NK cells
Several groups, including ours, have demonstrated in murine models that the antitumor activity of anti-CD137 agonistic mAbs is NK dependent.2,3,6 Based on the crucial role of NK cells in antitumor activity and the recognized expression of CD137 on IL-2–stimulated murine NK cells, we initially hypothesized that human NK cells cultured in IL-2 would express high levels of CD137 and that chimeric anti-CD137 mAbs would react with CD137 expressed on IL-2–stimulated human NK cells, inducing degranulation and cytokine release.
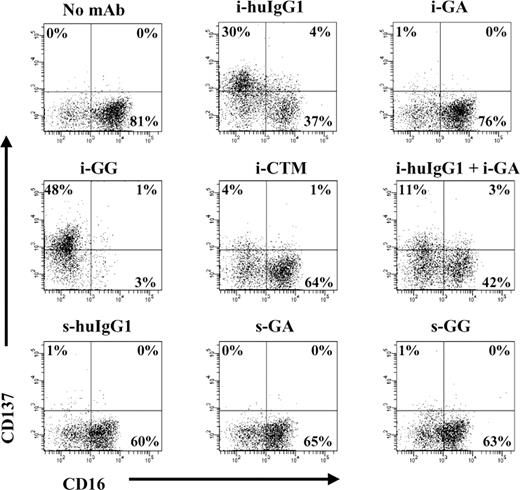
Interestingly, we found that after IL-2 stimulation and subsequent culture in RPMI 1640 supplemented with FBS, human NK cells did not demonstrate high levels of CD137 expression (data not shown). In contrast, IL-2–stimulated NK cells cultured in the presence of immobilized glycosylated mAbs showed high levels of CD137 expression (Figure 1). Expression of CD137 was transient and was not observed to a measurable extent after 72 hours of culture (data not shown). Of note, NK cells from one individual evidenced relatively high levels of CD137 after IL-2 stimulation. This exception may reflect ongoing infection/inflammation in this donor or individual variation in susceptibility for IL-2.
Immobilized glycosylated mAbs stimulate CD137 expression on human NK cells. IL-2–stimulated NK cells were cultured for 24 hours in the presence of immobilized or soluble chimeric mAbs as indicated. CD137 expression was measured by flow cytometry. Gates were set around CD56+ (NK) cells and numbers in the dot plots indicate percentage of CD56+ (NK) cells expressing CD137. Immobilized mAb culture conditions and soluble mAb culture conditions are indicated with i- and s-, respectively. The data shown are representative of 1 of 6 individual experiments. Statistical analysis is based on 6 experiments; CD137 expression levels are significantly increased in NK-cell cultures with i-huIgG1 or i-GG (P = .023 and P = .007, respectively) compared with i-GA cultures. HuIgG1 indicates polyclonal human IgG1; GA, aglycosylated chimeric anti-CD137 mAb; GG, glycosylated chimeric anti-CD137 mAb; and CTM, cetuximab.
Immobilized glycosylated mAbs stimulate CD137 expression on human NK cells. IL-2–stimulated NK cells were cultured for 24 hours in the presence of immobilized or soluble chimeric mAbs as indicated. CD137 expression was measured by flow cytometry. Gates were set around CD56+ (NK) cells and numbers in the dot plots indicate percentage of CD56+ (NK) cells expressing CD137. Immobilized mAb culture conditions and soluble mAb culture conditions are indicated with i- and s-, respectively. The data shown are representative of 1 of 6 individual experiments. Statistical analysis is based on 6 experiments; CD137 expression levels are significantly increased in NK-cell cultures with i-huIgG1 or i-GG (P = .023 and P = .007, respectively) compared with i-GA cultures. HuIgG1 indicates polyclonal human IgG1; GA, aglycosylated chimeric anti-CD137 mAb; GG, glycosylated chimeric anti-CD137 mAb; and CTM, cetuximab.
CD137 was enhanced only on IL-2–stimulated NK cells in cultures with immobilized GG (range: 22%-61%; P = .007) and not with GA (range: 1%-14%). Cultures of IL-2–stimulated NK cells in the presence of nonimmobilized (soluble) mAbs failed to induce CD137 expression. CD137 was also up-regulated on IL-2–stimulated NK cells cultured with immobilized polyclonal huIgG1 (range: 3%-73%; P = .023) and, to a lesser extent, with immobilized anti-EGFR mAb CTM (range: 3%-10%). The Fab region of GA and GG did not appear to augment CD137 expression, as the levels of CD137 in cultures with immobilized huIgG1 plus immobilized GA (range: 14%-25%) were lower than in cultures with immobilized huIgG1 or immobilized GA alone (range: 3%-73% and 1%-14%, respectively).
In addition, CD137 expression was inversely associated with the presence of FcγRIII. NK-cell cultures with high levels of CD137 showed low levels of FcγRIII, as indicated by CD16 in Figure 1. Likewise, cultures with low levels of CD137 were associated with high levels of FcγRIII. To avoid possible interference of purified chimeric anti-CD137 mAbs in flow cytometry analysis, CD137 expression levels were determined using commercial mouse anti–human CD137 mAb (clone 4B4-1), whose binding to CD137 was not blocked by 10-fold differences in concentra-tion of GA or GG mAb (range: 0.02 μg/mL to 20 μg/mL) as determined by flow cytometry–based competition-inhibition studies (data not shown).
To exclude other possible sources of NK-cell activation, we indirectly tested for the presence of LPS and evaluated the binding affinity of individual mAb preparations. The up-regulation of CD137 on the NK-cell surface was likely not induced by LPS contamination, because boiling the GG mAb preparation eliminated its ability to up-regulate CD137 (data not shown). Furthermore, observed differences in CD137 expression were also not likely to be secondary to variable levels of immobilized immunoglobulins because similar levels of immobilized GG, GA, and huIgG1 were present on the surface of precoated wells (data not shown). In addition, 10-fold increases in GG concentration did not further enhance CD137 expression on IL-2–stimulated NK cells. However, CD137 expression levels were slightly enhanced with a 10-fold increase in immobilized CTM concentration (10 μg/mL to 100 μg/mL) and CD137 started to reach expression levels as observed in cultures with 10 μg/mL GG (data not shown). These experiments support the conclusion that CD137 expression on NK cells is Fc dependent and that different Fc regions have variable ability to induce CD137 on human NK cells.
CD137 expression on NK cells is induced in an Fc-dependent fashion
The fact that immobilized glycosylated mAbs, but not soluble glycosylated mAbs or aglycoslyated mAbs, induce high levels of CD137 expression on IL-2–stimulated NK cells strongly supports the idea that CD137 expression on the NK-cell surface is mediated through Fc-FcγR interactions. To exclude the potential influence of a CD137-specific Fab region in non–Fc-mediated CD137 expression, purified Fab and Fc fragments of GG mAb were used in IL-2–stimulated NK-cell experiments.
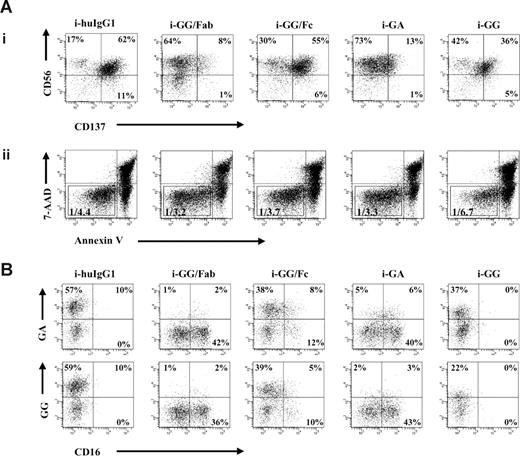
As expected, NK cells cultured with immobilized GG/Fc fragments induced CD137 expression at levels similar to those observed with immobilized GG mAb (range: 27%-61% and 22%-61%, respectively). In contrast, NK cells cultured with immobilized GA mAb or GG/Fab fragments induced only small amounts of CD137 expression (range: 1%-14% and 6%-13%, respectively), as shown in Figure 2Ai, and this difference was determined to be statistically significant (P = .001). Interestingly, the total number of live NK cells (gated as 7-AAD−/annexinV− cells) decreased in the culture with immobilized GG mAb versus the culture with purified GG/Fc fragments, as indicated by the increased ratio of total number of live cells versus total number of acquired cells, 1:6.7 and 1:3.7, respectively (Figure 2Aii).
CD137 expression on NK cells is induced in an Fc-dependent fashion. GG mAb was digested into separate Fab and Fc fragments using papain. Digests were passed over protein L or protein A columns, and single Fab fragments or Fc fragments were collected, respectively. IL-2–stimulated NK cells were cultured in the presence of immobilized chimeric mAb or immobilized fragments as indicated in the figure. (A) After 24 hours, CD137 expression was determined by flow cytometry. Gates were set around 7-AAD−/annexinV− cells. Numbers in the dot plots indicate the percentage of CD56+ (NK) cells expressing CD137 (i). Ratios represent the number of live NK cells and are calculated based on the total number of 7-AAD−/annexinV− cells within the total number of acquired cells (ii). (B) CD137 expressed on NK cells is recognized not only by commercial available mouse anti-CD137 mAb but also by both chimeric anti-CD137 mAbs, GA and GG. Experiment shown represents 1 of 3 individual experiments. Statistical analysis is based on 6 (i-GG and i-GA) and 3 (i-GG/Fc and i-GG/Fab) experiments; CD137 expression levels are significantly increased in NK-cell cultures with i-GG and i-GG/Fc compared with i-GA and i-GG/Fab (P = .001). HuIgG1 indicates polyclonal human IgG1; GA, aglycosylated chimeric anti-CD137 mAb; and GG, glycosylated chimeric anti-CD137 mAb.
CD137 expression on NK cells is induced in an Fc-dependent fashion. GG mAb was digested into separate Fab and Fc fragments using papain. Digests were passed over protein L or protein A columns, and single Fab fragments or Fc fragments were collected, respectively. IL-2–stimulated NK cells were cultured in the presence of immobilized chimeric mAb or immobilized fragments as indicated in the figure. (A) After 24 hours, CD137 expression was determined by flow cytometry. Gates were set around 7-AAD−/annexinV− cells. Numbers in the dot plots indicate the percentage of CD56+ (NK) cells expressing CD137 (i). Ratios represent the number of live NK cells and are calculated based on the total number of 7-AAD−/annexinV− cells within the total number of acquired cells (ii). (B) CD137 expressed on NK cells is recognized not only by commercial available mouse anti-CD137 mAb but also by both chimeric anti-CD137 mAbs, GA and GG. Experiment shown represents 1 of 3 individual experiments. Statistical analysis is based on 6 (i-GG and i-GA) and 3 (i-GG/Fc and i-GG/Fab) experiments; CD137 expression levels are significantly increased in NK-cell cultures with i-GG and i-GG/Fc compared with i-GA and i-GG/Fab (P = .001). HuIgG1 indicates polyclonal human IgG1; GA, aglycosylated chimeric anti-CD137 mAb; and GG, glycosylated chimeric anti-CD137 mAb.
CD137 was detected not only by commercial mouse anti-CD137 mAb but also by both chimeric anti-CD137 mAbs, demonstrating that GG and GA cross-react with CD137 induced on IL-2–stimulated NK cells. These data have importance for future clinical translational studies (Figure 2B). Importantly, the purity of the Fab fragments was 95% and of the Fc fragments, 75%. However, given that the Fab fragments did not induce high levels of CD137, it is unlikely that our results are secondary to contamination. Therefore, these data provide convincing evidence that CD137 is induced on IL-2–stimulated NK cells in an Fc-dependent fashion.
Chimeric GA and GG mAbs have similar Fab regions despite differences in Fc
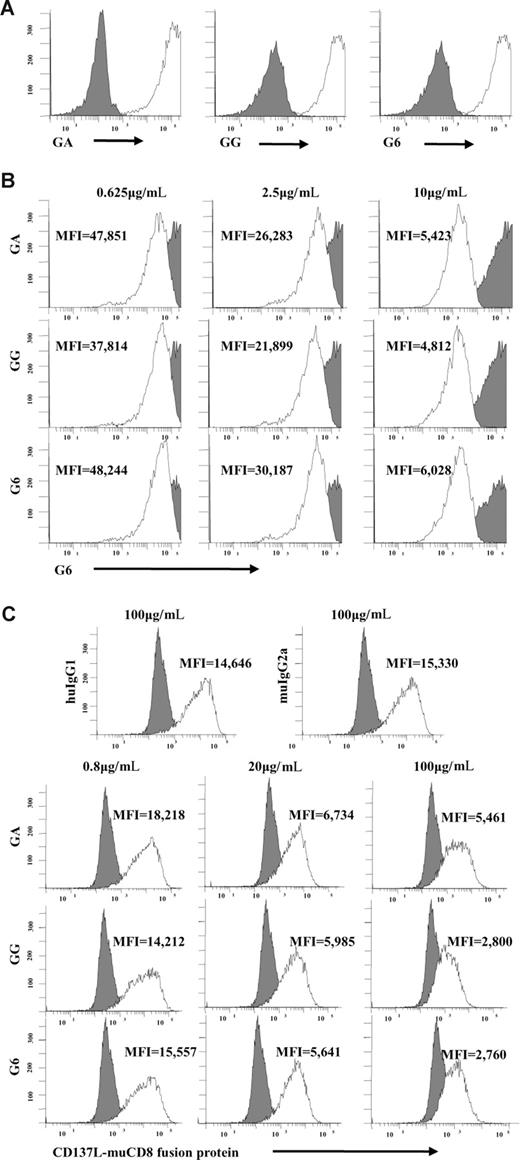
To further evaluate the potential role of the Fab fragments of GA and GG in inducing CD137 on the NK-cell surface, we compared their ability to bind 4-1BB transfected CHO cells and to block natural 4-1BB-4-1BBL interactions. Both mAbs showed similar binding to CHO/CD137 cells and failed to bind wild-type (CHO/WT) controls (Figure 3A). To determine whether the parent mAb (mouse antihuman CD137) G6 and both chimeric mAbs GA and GG recognized the same CD137 epitope, CHO/CD137 cells were incubated with increasing concentrations of purified G6, GA, or GG mAb, respectively (Figure 3B). All mAbs partially blocked binding of G6-APC in a dose-dependent fashion, suggesting that chimerization did not alter epitope affinity. Finally, to further define the functional characteristics of these mAbs, the ability of G6, GA, and GG to block the interaction of the CD137 receptor with its natural ligand, CD137L, was determined. CHO/CD137 cells, incubated with increasing concentrations of purified G6, GA, or GG, blocked the binding of human CD137L/mouse CD8 fusion protein in a dose-dependent fashion (Figure 3C). These data indicate that both chimeric mAbs bind similarly to CD137 and partially block CD137 receptor-ligand interactions in a dose-dependent fashion, suggesting equal specificity of their Fab region for the CD137 receptor. Thus, the fundamental difference between GA and GG mAbs is the status of N-glycosylation of the mAb Fc region. The fact that the Fab fragments of these antibodies are identical suggests that differences in CD137 expression levels in NK-cell cultures with immobilized GG and GA mAb are secondary to the variable ability of the Fc regions of GG and GA to interact with putative FcγRs expressed on human NK cells.
Chimeric GA and GG mAbs have similar Fab regions despite differences in Fc. (A) G6, GA, and GG mAbs bind similar to CD137-expressing CHO cells and fail to bind CHO/WT controls. (B) CD137-expressing CHO cells were incubated with different concentrations of purified G6, GA, or GG mAbs. Cells were subsequently stained with G6-APC mAb. Increasing concentrations of purified G6, GA, or GG mAbs blocked binding of G6-APC as indicated by MFI in a dose-dependent fashion. (C) CD137-expressing CHO cells were first incubated with increasing concentrations of G6, GA, or GG and subsequently incubated with human CD137L-muCD8 fusion protein. Cells were then stained with PE-conjugated anti-muCD8. As indicated by MFI, G6, GA, and GG blocked CD137/CD137L interaction in a dose-dependent fashion. Filled histograms represent CHO/WT cells (A) or isotype control mAb (B,C). Open histograms represent staining with the indicated antibody. GA indicates aglycosylated chimeric anti-CD137 mAb; GG, glycosylated chimeric anti-CD137 mAb; G6, mouse antihuman CD137 mAb; and MFI, mean fluorescence intensity.
Chimeric GA and GG mAbs have similar Fab regions despite differences in Fc. (A) G6, GA, and GG mAbs bind similar to CD137-expressing CHO cells and fail to bind CHO/WT controls. (B) CD137-expressing CHO cells were incubated with different concentrations of purified G6, GA, or GG mAbs. Cells were subsequently stained with G6-APC mAb. Increasing concentrations of purified G6, GA, or GG mAbs blocked binding of G6-APC as indicated by MFI in a dose-dependent fashion. (C) CD137-expressing CHO cells were first incubated with increasing concentrations of G6, GA, or GG and subsequently incubated with human CD137L-muCD8 fusion protein. Cells were then stained with PE-conjugated anti-muCD8. As indicated by MFI, G6, GA, and GG blocked CD137/CD137L interaction in a dose-dependent fashion. Filled histograms represent CHO/WT cells (A) or isotype control mAb (B,C). Open histograms represent staining with the indicated antibody. GA indicates aglycosylated chimeric anti-CD137 mAb; GG, glycosylated chimeric anti-CD137 mAb; G6, mouse antihuman CD137 mAb; and MFI, mean fluorescence intensity.
CD137 expression levels on NK cells are associated with defined patterns of Fc glycosylation
Human IgG1 mAbs carry a conserved N-glycan at Asn297 of the Fc region. Fc-FcγR interactions are dependent on the extent of core fucosylation of the Fc N-glycan, with lower fucosylated IgG1 having significantly enhanced affinity for the FcγIII receptor in comparison with those with high fucose content. The level of fucosylation has functional implications, as lower fucosylated mAbs induce more potent antibody-dependent cellular cytotoxicity (ADCC) at lower antigen densities.13 Based on our observations that mAbs from different sources stimulated variable levels of CD137 on the NK-cell surface, we sought to investigate whether the difference in fucose composition of N-glycans was associated with CD137 expression.
To quantitate the monosaccharide composition, we performed HPAEC profile analysis of hydrolysates of the different mAbs studied. To exclude the interference of potential glycosylation at other sites, we purified respective IgG1-Fc fragments from all mAbs and analyzed the monosaccharide composition of the Fc N-glycan. Treatment of the mAb Fc fragments with trifluoroacetic acid resulted in complete hydrolysis of N-glycan into the compositional monosaccharides, where N-acetylglucosamine (GlcNAc) was released as glucosamine (GlcN). The HPAEC profiles of the hydrolysates of all IgG1-Fc fragments are demonstrated in Figure 4.
HPAEC profile analysis of IgG1-Fc. Trifluoroacetic (TFA) hydrolysis resulted in complete degradation of the N-glycan within the separate Fc fragments to compositional monosaccharides where N-acetylglucosamine (GlcNac) was released as glucosamine. Compositional monosaccharide patterns were compared with a standard monosaccharide profile composed of fucose, glucosamine, galactose, glucose, and mannose, respectively. Fuc indicates fucose; GlcN, glucosamine; Gal, galactose; Glc, glucose; Man, mannose; GP-2, standard glycopeptide; GA, aglycosylated chimeric anti-CD137 mAb; GG, glycosylated chimeric anti-CD137 mAb; CTM, cetuximab; and HuIgG1, polyclonal human IgG1.
HPAEC profile analysis of IgG1-Fc. Trifluoroacetic (TFA) hydrolysis resulted in complete degradation of the N-glycan within the separate Fc fragments to compositional monosaccharides where N-acetylglucosamine (GlcNac) was released as glucosamine. Compositional monosaccharide patterns were compared with a standard monosaccharide profile composed of fucose, glucosamine, galactose, glucose, and mannose, respectively. Fuc indicates fucose; GlcN, glucosamine; Gal, galactose; Glc, glucose; Man, mannose; GP-2, standard glycopeptide; GA, aglycosylated chimeric anti-CD137 mAb; GG, glycosylated chimeric anti-CD137 mAb; CTM, cetuximab; and HuIgG1, polyclonal human IgG1.
As expected, the hydrolysis of GA did not release typical monosaccharides (eg, mannose [Man], GlcN, and galactose [Gal]) of the N-glycan, confirming that no N-glycan was attached to this antibody. In contrast, GG, CTM, and huIgG1 revealed typical monosaccharide patterns compared with a standard monosaccharide profile.
For quantification, the peaks corresponding to the different monosaccharides were integrated and peak areas were normalized to molar quantity by comparison with a standard glycopeptide (GP-2) isolated from hen's egg yolk that contains a full-size biantennary complex–type N-glycan with the following molar ratios of monosaccharides: Man/Gal/GlcN = 3:2:4. Based on these calculations, the molar ratios of the monosaccharide composition of all IgG1-Fc fragments are summarized in Table 1. GG showed much less fucosylated N-glycan than CTM and huIgG1. Interestingly, CTM showed a higher content of GlcNAc (Man/GlcN = 3:5) than GG and huIgG1 (Man/GlcN = 3:4), suggesting that this mAb may have an additional bisecting GlcNAc. These observations suggest that the low fucose content on GG might contribute to its enhanced ability to induce CD137 on human NK cells.
Compositional monosaccharide quantification of IgG1-Fc
| IgG1-Fc . | Monosaccharide molar ratios determined by HPAEC-PAD analysis* . | |||
|---|---|---|---|---|
| Fuc . | GlcN . | Gal . | Man† . | |
| Biantennary GP‡ | na | 3.86 | 2.08 | 3.00 |
| HuIgG1 | 0.95 | 3.50 | 0.80 | 3.00 |
| GG | 0.56 | 3.60 | 1.40 | 3.00 |
| GA | na | na | na | na |
| CTM | 1.10 | 5.20 | 1.90 | 3.00 |
| IgG1-Fc . | Monosaccharide molar ratios determined by HPAEC-PAD analysis* . | |||
|---|---|---|---|---|
| Fuc . | GlcN . | Gal . | Man† . | |
| Biantennary GP‡ | na | 3.86 | 2.08 | 3.00 |
| HuIgG1 | 0.95 | 3.50 | 0.80 | 3.00 |
| GG | 0.56 | 3.60 | 1.40 | 3.00 |
| GA | na | na | na | na |
| CTM | 1.10 | 5.20 | 1.90 | 3.00 |
PAD indicates pulsed amperometric detection; Fuc, fucose; GlcN, glucosamine; Gal, galactose; Man, mannose; HuIgG1, polyclonal human IgG1; GG, glycosylated chimeric anti-CD137 mAb; GA, aglycosylated chimeric anti-CD137 mAb; CTM, cetuximab; and na, not applicable.
Fc fragments were hydrolyzed in 2 M trifluoroacetic acid (TFA) at 100°C for 4 hours. The hydrolysates were lyophilized and subject to Dionex HPAEC-PAD analysis.
For quantification, the peaks corresponding to the different monosaccharides were integrated and peak areas were normalized to molar quantity by comparison with biantennary GP. The molar ratios were normalized by setting mannose as 3.
Biantennary GP represents a standard glycopeptide isolated from hen's egg yolk that contains a full-size biantennary complex–type N-glycan with the following molar ratios of monosaccharides: Man/Gal/GlcN = 3:2:4.
CD137 expression on human NK cells is associated with phenotypic markers of activation and the release of proinflammatory cytokines
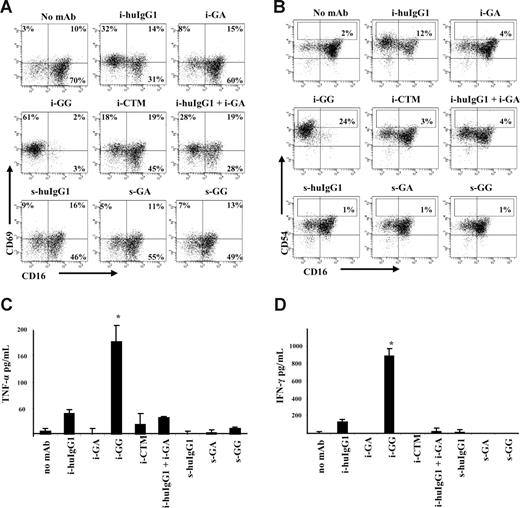
To begin to understand the function of CD137 on human NK cells, we sought to associate CD137 expression with cellular activation, as characterized by NK-cell surface expression of CD69 and CD54 and proinflammatory cytokine secretion. CD137-expressing NK- cell cultures were associated with increased levels of CD69 and CD54bright expression, with the highest levels of CD69 expression in cultures with huIgG1 and GG mAb (range: 32%-62% and 36%-86%, respectively; P = .036 for GG). CD54bright expression varied from 4% to 17% expression in cultures with huIgG1 (P = .019) to 10% to 24% expression in cultures with GG mAb (P = .004), as indicated in Figure 5A,B.
CD137 expression on human NK cells associates with phenotypic markers of activation and proinflammatory cytokine secretion. Gates were set around CD56+ (NK) cells and numbers in the dot plots indicate the percentage of NK cells expressing indicated surface marker. CD137 expression on IL-2–stimulated NK cells associates with increased levels of CD69 (A) and CD54bright (B) expression in cultures with immobilized huIgG1 and GG mAb. Proinflammatory cytokines were determined by ELISA in supernatants after 24 hours of culture. (C) TNF-α levels were significantly (*P = .047) increased in cultures with immobilized GG mAb. (D) IFN-γ levels were significantly (*P = .048) increased in cultures with immobilized GG mAb. Immobilized mAb culture conditions and soluble mAb culture conditions are indicated with i- and s-, respectively. Experiment shown represents 1 of 6 individual experiments. Statistical analysis is based on 6 experiments; CD69 expression levels are significantly increased in NK-cell cultures with i-GG (P = .036) and CD54bright expression levels are significantly increased in NK-cell cultures with i-huIgG1 (P = .019) and i-GG (P = .004) compared with i-GA. HuIgG1 indicates polyclonal human IgG1; GA, aglycosylated chimeric anti-CD137 mAb; GG, glycosylated chimeric anti-CD137 mAb; and CTM, cetuximab. Error bars represent SD.
CD137 expression on human NK cells associates with phenotypic markers of activation and proinflammatory cytokine secretion. Gates were set around CD56+ (NK) cells and numbers in the dot plots indicate the percentage of NK cells expressing indicated surface marker. CD137 expression on IL-2–stimulated NK cells associates with increased levels of CD69 (A) and CD54bright (B) expression in cultures with immobilized huIgG1 and GG mAb. Proinflammatory cytokines were determined by ELISA in supernatants after 24 hours of culture. (C) TNF-α levels were significantly (*P = .047) increased in cultures with immobilized GG mAb. (D) IFN-γ levels were significantly (*P = .048) increased in cultures with immobilized GG mAb. Immobilized mAb culture conditions and soluble mAb culture conditions are indicated with i- and s-, respectively. Experiment shown represents 1 of 6 individual experiments. Statistical analysis is based on 6 experiments; CD69 expression levels are significantly increased in NK-cell cultures with i-GG (P = .036) and CD54bright expression levels are significantly increased in NK-cell cultures with i-huIgG1 (P = .019) and i-GG (P = .004) compared with i-GA. HuIgG1 indicates polyclonal human IgG1; GA, aglycosylated chimeric anti-CD137 mAb; GG, glycosylated chimeric anti-CD137 mAb; and CTM, cetuximab. Error bars represent SD.
Because activated human NK cells are known to secrete proinflammatory cytokines, we evaluated the supernatants of NK- cell cultures for IFN-γ and TNF-α. Levels of TNF-α and IFN-γ in cultures containing immobilized GG were significantly increased (P = .047 and P = .048, respectively) in comparison with cultures with immobilized huIgG1, CTM, or soluble mAb (Figure 5C,D). The levels of IFN-γ and TNF-α declined over time—remaining detectable only in wells with immobilized GG after 72 hours (data not shown). In conclusion, these data suggest that Fc-dependent CD137 expression is associated with phenotypic markers of activation and proinflammatory cytokine release.
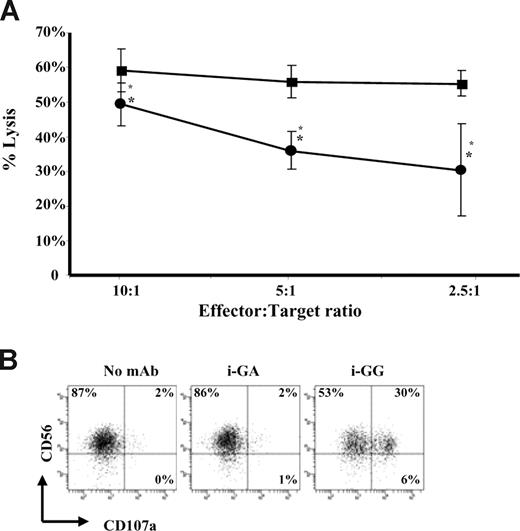
Degranulation precedes CD137 expression on human NK cells and results in less efficient lysis of NK-sensitive tumor targets by CD137-expressing human NK cells
Although Fc-FcγRIII interactions induce NK-cell activation resulting in the up-regulation of phenotypic markers of activation and the release of proinflammatory cytokines, they do not reveal the capability of these NK cells to lyse targets cells. 51Cr-release assays were used to determine the lytic capacity of CD137-expressing and non–CD137-expressing NK cells. Surprisingly, the lytic capacity of CD137-expressing NK cells was significantly diminished (P = .005) in comparison with non–CD137-expressing NK cells (Figure 6A). Importantly, influence on lytic capacity mediated through CD137-CD137L interactions is unlikely given the fact that K562 tumor targets did not express CD137L as determined by flow cytometry (data not shown). To understand this observation, the temporal relationship between CD137 expression and NK-cell degranulation, as measured by CD107a expression,19 was determined. We observed that in the presence of immobilized GG mAb, IL-2–stimulated NK cells express high levels of the cytolytic surface marker CD107a (range: 17%-43%; P = .011 compared with immobilized GA) after 6 hours of culture (Figure 6B), whereas CD137 cell surface expression peaks between 12 to 24 hours of culture. These data suggest that degranulation and CD137 expression on human NK cells are discordant, which is reflected in the lytic activity against K562 tumor targets.
CD137-expressing human NK cells lyse NK-sensitive tumor targets less efficiently than non–CD137-expressing human NK cells. (A) IL-2–stimulated NK cells were cultured for 24 hours in the presence of immobilized GG mAb (●) or immobilized GA mAb (■) and subsequently used in a 4-hour 51Cr-release assay to determine lytic activity against K562 target cells. Non–CD137-expressing NK cells (■) and CD137 expressing NK cells (●) were added at the indicated effector-to-target ratios (E/T). Data represent mean and standard deviation of 2 independent experiments. Lytic activity of CD137-expressing NK cells (i-GG cultured) was significantly diminished compared with non–CD137-expressing NK cells (i-GA cultured) with *P = .005. Error bars represent SD. (B) Expression of degranulation marker CD107a on IL-2–stimulated NK cells after 6 hours of culture with i-GG. Gates were set around live cells based on forward and site scatter plots and numbers in the dot plots indicate the percentage of NK cells expressing CD107a. Experiment shown represents 1 of 4 individual experiments. Statistical analysis is based on 4 experiments performed with P = .011 for i-GG compared with i-GA. Immobilized mAb culture conditions are indicated with i-. GG indicates glycosylated chimeric anti-CD137 mAb; GA, aglycosylated chimeric anti-CD137 mAb.
CD137-expressing human NK cells lyse NK-sensitive tumor targets less efficiently than non–CD137-expressing human NK cells. (A) IL-2–stimulated NK cells were cultured for 24 hours in the presence of immobilized GG mAb (●) or immobilized GA mAb (■) and subsequently used in a 4-hour 51Cr-release assay to determine lytic activity against K562 target cells. Non–CD137-expressing NK cells (■) and CD137 expressing NK cells (●) were added at the indicated effector-to-target ratios (E/T). Data represent mean and standard deviation of 2 independent experiments. Lytic activity of CD137-expressing NK cells (i-GG cultured) was significantly diminished compared with non–CD137-expressing NK cells (i-GA cultured) with *P = .005. Error bars represent SD. (B) Expression of degranulation marker CD107a on IL-2–stimulated NK cells after 6 hours of culture with i-GG. Gates were set around live cells based on forward and site scatter plots and numbers in the dot plots indicate the percentage of NK cells expressing CD107a. Experiment shown represents 1 of 4 individual experiments. Statistical analysis is based on 4 experiments performed with P = .011 for i-GG compared with i-GA. Immobilized mAb culture conditions are indicated with i-. GG indicates glycosylated chimeric anti-CD137 mAb; GA, aglycosylated chimeric anti-CD137 mAb.
Discussion
The critical findings of these studies are that (1) immobilized Fc enhances the expression of CD137 on IL-2–stimulated NK cells, (2) the levels of CD137 expression are dependent on patterns of Fc glycosylation known to impact Fc interactions with FcγRIII, and (3) the ability to induce CD137 expression is independent of the antigen specificity of the Fab region. Importantly, our experiments do not specifically address the therapeutic utility of targeting the CD137 pathway on CD137-expressing NK cells, which is perhaps best studied using CD137L. Our findings shed new light onto a secondary function of mAbs against costimulatory molecules and demand caution when describing mAbs as being “agonistic” if their function in in vivo and in vitro studies is assessed with intact Fc regions.
Knowledge of the functional import of CD137 on human NK cells is primarily derived from murine studies. It is now clear that IL-2–stimulated murine NK cells express CD137 in vivo and that, in some but not all cases, these cells are required for the antitumor effects of agonistic mAbs against CD137.2,3,6 The function of CD137/CD137L interactions on murine NK cells is not completely understood, yet it appears that CD137 ligation enhances NK-cell proliferation and cytokine secretion, and may provide a helper function in the induction of cytotoxic T lymphocytes (CTLs), thereby bridging the innate and adaptive immune system in mice.20,21
Correlate studies suggest that human NK cells play a pivotal role in the regulation of both innate and adaptive immune responses, yet limited information is available regarding CD137 expression and its role in modulating these interactions.22 For example, it has been demonstrated that low levels of CD137 (∼ 2.02%) can be induced on NKB1+ cells when PBMCs are exposed to doxorubicin, bleomycin, or mitomycin, although it remains uncertain whether the small NKB1+CD137+ cell population depicted in this study represents T cells, NK cells, NKT cells, or γδT cells.23 Similarly, from a functional perspective, human NK cells transfected with a construct containing the intracellular domain of CD137 directly linked to an anti-CD3/CD19 chimeric receptor are more effective than NK cells transfected with anti–CD3-CD19 chimeric receptor alone, in both killing leukemic cells and producing IFN-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF).24
Our experiments using various chimeric IgG1 antibodies with different Fc regions support the novel concept that interactions between Fc and putative FcγRs on the surface of human NK cells directly modulate the CD137 costimulatory pathway independent of mAb antigen specificity. Because NK cells express CD16 and CD32 exclusively, it is likely that the observed effects are secondary to ligation of these receptors.25 Importantly, it is unlikely that the ability of GG and GA mAbs to block CD137-CD137L interactions influenced our results, as CD137L is reported to be expressed only on activated macrophages, dendritic cells, and B cells and not on human NK cells.3,26 In addition, similar CD137 expression levels were observed in NK-cell cultures in which the CD137-specific Fab fragment was absent (NK-cell cultures with GG/Fc). Although the up-regulation of CD137 did not appear to be augmented by cross-reactivity of anti-CD137 specific Fab regions, these differences are difficult to ascertain in vitro. Our findings offer new insight into the role of antibody Fc fragments in mediating CD137-based NK-cell responses.
Consistent with the idea that Fc-FcγR interactions play an important role in regulating CD137 expression on human NK cells, we found that for GG and CTM, the levels of CD137 expression were inversely associated with fucose content within the N-glycan of the Fc region. Furthermore, whereas CD137 expression levels were enhanced in the presence of 10-fold increases in CTM concentration, CD137 expression levels did not increase in the presence of immobilized GG at concentrations higher than 10 μg/mL. These data are consistent with previous observations that low-fucose IgG1 mAbs enhance NK-cell activation at lower mAb density.13 Interestingly, we did not observe an inverse correlation between CD137 expression levels and fucosylated N-glycan in cultures with huIgG1. This observation may reflect the fact that the huIgG1 used in our experiments is polyclonal, resulting in potential enhancement of CD137 expression levels through Fab binding.
The higher GlcNAc contents in CTM (Man/GlcN = 3:5) in comparison with GG and huIgG1 (Man/GlcN = 3:4) implicates the presence of a bisecting GlcNAc in the N-glycan of this mAb. It is reported that the addition of bisecting GlcNAc enhances ADCC much less efficiently than the presence or absence of fucose.27 Our observation that, regardless of the higher GlcNAc content, CTM is less efficient in inducing CD137, phenotypic markers of activation, and proinflammatory cytokines is in agreement with these previously published reports.
Previous studies have demonstrated that Fc-FcγRIII cross-linking induces NK-cell activation resulting in the loss of FcγRIII receptor expression and cytokine release10 (eg, IFN-γ and TNF-α). We found a direct association between GG cross-linking and acquisition of an activated NK-cell phenotype characterized by CD69 and CD54bright expression. This pattern of activation was inversely associated with FcγRIII expression. However, our studies did not rule out that possible FcγRIII loss could have been artifactual, secondary to the interference of IgG1 with FcγRIII binding.
Interestingly, the number of live NK cells was substantially decreased in cultures with GG. This observation is likely secondary to the specificity of GG for CD137 and 2 potential mechanisms may be involved. First, GG interaction with CD137 may induce ADCC among CD137-expressing NK cells and/or, second, GG interactions may either block or deliver a signal through CD137. These mechanisms are not mutually exclusive, and both could potentially play a role.
In addition to displaying phenotypic markers characteristic of NK activation, cultures with high levels of CD137 expression demonstrated the proinflammatory cytokines IFN-γ and TNF-α. Furthermore, GG-stimulated NK cells were functionally capable of degranulation as evidenced by high levels of CD107a expression.28 As degranulation precedes CD137 expression, reflected by diminished lytic capacity against NK-sensitive tumor targets, this may suggest that CD137-expressing human NK cells play a role in immunomodulation rather than exhibiting direct lytic functions. Importantly, because there is a substantial time interval between the intracellular expression of CD107a and cytokines (maximum at6 hours) and the surface expression of CD137 (maximum at > 12 hours), we cannot ascertain whether CD137-expressing cells are directly responsible for the observed differences.
In summary, our findings that immobilized Fc induces CD137 expression on IL-2–stimulated NK cells are pivotal to the interpretation of studies using anti-CD137 mAbs with an Fc region capable of binding FcγRs. Specifically, under these circumstances, effects previously attributed to CD137 interactions with the Fab region, must now be reevaluated as potentially related to Fc-induced expression of CD137 on NK cells with subsequent effects due to (1) direct interaction of CD137L with Fc-induced CD137, (2) direct stimulation of Fc-induced CD137 by agonistic anti-CD137 mAbs, or (3) antibody blockade of interactions between Fc-induced CD137 and CD137L.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was partially supported by National Institutes of Health (Bethesda, MD) grant 5R44CA107608-04 and by an Small Business Innovation Research grant from the National Cancer Institute, Bethesda, MD, on which S.E.S. and L.C. are subcontractors.
National Institutes of Health
Authorship
Contribution: W.L., X.Z., A.W., E.B., and Y.W. performed experiments and analyzed data; C.J.V. designed and performed experiments, analyzed data, and wrote the paper; L.C. provided transfectants and the mouse antihuman anti-CD137 monoclonal antibody; D.G.S. provided the chimeric anti-CD137 monoclonal antibodies; G.T. performed statistical analysis; L.-X.W., K.T, D.H.S., D.M., and S.E.S. designed experiments, and reviewed data and the paper.
Conflict-of-interest disclosure: S.E.S. and L.C. receive royalties from GTC Biotherapeutics through the Mayo Clinic College of Medicine for licensure of intellectual property related to CD137. S.E.S. is a cofounder and major stockholder in Gliknik, a biotechnology company. D.G.S. is employed by and owns stock in GTC Biotherapeutics. All other authors declare no competing financial interests.
Correspondence: Scott E. Strome, Department of Otorhinolaryngology-Head and Neck Surgery, University of Maryland, 16 South Eutaw Street, Suite 500, Baltimore, MD 21201-168; e-mail: sstrome@smail.umaryland.edu.
References
Author notes
*W.L. and C.J.V. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal