Abstract
We report the retrospective outcomes of unrelated donor (URD) transplants in 169 patients with acute lymphoblastic leukemia (ALL) in first complete remission (CR1) who received transplants between 1995 and 2004. Median age was 33 years (range, 16-59 years). A total of 50% had a white blood cell count (WBC) more than 30 × 109/L, 18% extramedullary disease, 42% achieved CR more than 8 weeks from diagnosis, 25% had adverse cytogenetics, and 19% had T-cell leukemia. A total of 41% were HLA well-matched, 41% partially matched with their donors, and 18% were HLA-mismatched. At 54-month median follow-up, incidences of acute grade 2-IV, III to IV, and chronic graft-versus-host disease were 50%, 25%, and 43%, respectively. Five-year treatment-related mortality (TRM), relapse, and overall survival were 42%, 20%, and 39%, respectively. In multivariate analyses, TRM was significantly higher with HLA-mismatched donors and T-cell depletion. Relapse risk was higher if the diagnostic WBC was more than 100 × 109/L. Factors associated with poorer survival included WBC more than 100 × 109/L, more than 8 weeks to CR1, cytomegalovirus seropositivity, HLA mismatching, and T-cell depletion. Nearly 40% of adults with ALL in CR1 survive 5 years after URD transplantation. Relapse risks were modest; TRM is the major cause of treatment failure. Selecting closely HLA-matched URD and reducing TRM should improve results.
Introduction
The outcome of adults with acute lymphoblastic leukemia (ALL) remains disappointing. A large prospective trial by the Medical Research Council (MRC) and the Eastern Cooperative Oncology Group (ECOG), including more than 2000 patients accrued over 13 years, recently concluded and resulted in 38% 5-year disease-free survival (DFS).1,2 This trial had upfront sibling allografting for all patients in first complete remission (CR1) irrespective of risk status. Patients who had sibling allografts in CR1 enjoyed more than 50% prolonged DFS, which was superior to that of patients treated with chemotherapy alone using a donor versus no donor analysis. Other trials3,4 have previously suggested that sibling allografting in CR1 produces superior outcomes to chemotherapy or autograft, but this strategy has not gained universal acceptance. A meta-analysis of all randomized studies indicated a 25% better survival in the sibling donor group and a more than 40% advantage with transplantation in high-risk patients.5
The MRC-ECOG study1 and other recent studies have better defined the risk factors for treatment failure with chemotherapy, thus identifying the subset of patients requiring a different approach if the outcome of this disease is to improve substantially. These risk factors include a high white blood cell count (WBC) at diagnosis (> 30 × 109/L in B-cell disease, > 100 × 109/L in T-cell disease), age more than 35 years, adverse cytogenetics (including the new category of > 5 abnormalities),6 and various indicators of initial chemosensitivity and disease response. Interestingly, the MRC-ECOG study did not show that time to CR affected survival, but there are now increasing data that the presence of minimal residual disease (MRD) at certain early time points has a profound influence on subsequent outcome. A large prospective German study,7 which analyzed MRD using quantitative molecular techniques at 9 time points in the first year, showed that patients with no detectable MRD had a 66% 3-year DFS compared with 12% in those with more than 10−4 level of MRD.
Based on the evidence that sibling allografting may be the best strategy in high-risk adult ALL, many investigators have hypothesized that allografting using unrelated donors (URDs) may also produce improved survival. Recent German studies support this view. A comparison of 38 patients who underwent URD stem cell transplantation (SCT) for ALL with 46 patients with related donors showed similar survival (44% vs 46%, P = not significant) and no difference in treatment-related mortality (TRM).8 Another study of 99 patients who underwent URD SCT for ALL reported a modest TRM of 31% in a multicenter setting.9
Forty percent to 50% of adults and children who received transplants using URD in second CR experience prolonged DFS.10,11 URD transplantation is an accepted strategy in Philadelphia chromosome–positive (Ph+) ALL in CR1 in adults, and the results are clearly superior to those achieved with chemotherapy.12 Using the Center for International Blood and Marrow Transplant Research (CIBMTR) database, we retrospectively analyzed the outcome of URD SCT in CR1 patients with Ph− ALL. As a retrospective analysis of registry data, the data are dependent on reporting and may be affected by other selection biases. We elected to include patients 16 to 21 years of age in the analysis but recognize that many of these patients are now treated with pediatric protocols. We hypothesized that a substantial proportion of adult patients (> 16 years) with Ph− ALL would experience prolonged DFS after URD SCT performed in CR1 and aimed to identify the pretransplantation patient-, disease-, and transplantation-related prognostic factors that affect the probability of survival.
Methods
Data sources
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry, Autologous Blood and Marrow Transplant Registry, and the National Marrow Donor Program (NMDP) that comprises a voluntary working group of more than 450 transplantation centers worldwide that contributes detailed data on consecutive allogeneic and autologous hematopoietic SCT to a Statistical Center at the Medical College of Wisconsin in Milwaukee, and the NMDP Coordinating Center in Minneapolis, MN. Participating centers are required to report all transplantations consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for errors, physicians' review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are done so with a waiver of informed consent and in compliance with Health Insurance Portability and Accountability Act regulations as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
The CIBMTR collects data at 2 levels: registration and research. Registration data include disease type, age, sex, pretransplantation disease stage and chemotherapy responsiveness, date of diagnosis, graft type (bone marrow- and/or blood-derived stem cells), high-dose conditioning regimen, posttransplantation disease progression and survival, development of a new malignancy, and cause of death. Requests for data on progression or death for registered patients are at 6-month intervals. All CIBMTR teams contribute registration data. More detailed comprehensive research data are collected on a subset of registered patients selected using a weighted randomization scheme and include detailed disease and pretransplantation and posttransplantation clinical information.
Patient identification and eligibility
A total of 1109 patients were registered as having undergone an URD SCT for adult patients (16 years of age or older) in CR1 for ALL between 1995 and 2004. Of these, comprehensive research patient, disease, and transplantation characteristics were available on 562 (51%) patients. Patient, disease, and transplantation characteristics and overall survival of those with or without comprehensive data were similar (overall survival at 5 years, 42% [range, 39%-46%] vs 40% [range, 35%-44%], respectively, P = .43). Patients with Ph+ ALL (n = 254), unknown cytogenetic abnormality (n = 100), L3 ALL (n = 12), cord blood transplants (n = 7), and patients whose time from CR1 to transplantation was 12 months or more (n = 16) were excluded from the analyses. A total of 169 patients met the on-study criteria. The cases identified came from 85 reporting centers from 17 different countries. Median follow-up of survivors was 54 months (range, 3-133 months).
Definition of HLA matching
A new human leukocyte antigen (HLA) classification was used for CIBMTR studies and is based on the “best available typing.” This includes retrospective data from the NMDP high-resolution typing project plus any other allele-level typing provided by the transplantation centers. “Well matched” was defined as no known disparity at HLA A, B, C, DRB1, “partially matched” as one locus known or likely disparity with their donors, and “mismatched” as more than or equal to 2 locus disparities.13 Forty-one percent were well matched (no known disparity at HLA A, B, C, DR) and 41% partially matched (1 locus known or likely disparity) with their donors; these are analyzed together because of similar outcomes (see “Treatment-related mortality”). Eighteen percent of patients were defined as mismatched.
Endpoints
Primary endpoints were TRM, graft-versus-host disease (GVHD), leukemia relapse (hematologic and extramedullary), DFS, and overall survival. TRM was defined as death during continuous CR. Relapse was defined as hematologic leukemia recurrence. For analyses of DFS, treatment failure was leukemia relapses or deaths from any cause; patients alive and in CR were censored at the time of last follow-up. For analyses of overall survival, failure was death from any cause; surviving patients were censored at the date of last contact. The date of the transplantation was the starting time point for calculating all outcomes.
Statistical methods
Univariate probabilities of DFS and overall survival were calculated using the Kaplan-Meier estimator, with SE estimated by Greenwood's formula. Ninety-five percent confidence intervals (CIs) were calculated using log-transformed intervals. Probabilities of TRM and leukemia relapse were calculated using cumulative incidence curves to accommodate competing risks.14 Potential prognostic factors for outcomes of interest were evaluated in a multivariate analysis using Cox proportional hazards regression.15 The variables considered in the multivariate analysis were: age at transplantation (≤ 20 years vs 21-30 years vs 31-40 years vs 41-50 years vs 51-60 years), sex, Karnofsky performance status pretransplantation (< 90% vs ≥ 90%), lineage (T vs B), WBC at diagnosis (≤ 100 × 109/L vs > 100 × 109/L), time from diagnosis to CR1 (≤ 8 weeks vs > 8 weeks), time from diagnosis to transplantation (≤ 6 months vs > 6 months), time from CR1 to transplantation (< 3 months vs 3-6 months vs > 6 months), extramedullary disease (no vs yes), cytogenetics (no abnormalities vs t(4;11) ± others/hypodiploid ± others or ≥ 5 abnormalities vs others), conditioning regimen (cyclophosphamide + total body irradiation [TBI] ± other vs other), dose of TBI (< 1300 cGy vs > 1300 cGy), dose of cyclophosphamide (< 100 mg/kg vs 100-120 mg/kg vs ≥ 120 mg/kg), donor age (≤ 30 years vs 31-40 years vs > 40 years), donor-recipient sex match (male-male vs male-female vs female-male vs female-female), donor-recipient cytomegalovirus (CMV) status (−/− vs +/+ vs +/− vs −/+), donor-recipient HLA match (well matched vs partially matched vs mismatched), graft type (bone marrow vs peripheral blood stem cells), year of transplantation (1995-2000 vs 2001-2004), and T-cell depletion (TCD; no vs yes). All computations were made using the proportional hazards regression procedure in the statistical package of SAS, version 9. Forward stepwise variable selection at a 0.05 significance level was used to identify covariates associated with the main outcome. In the model, the assumption of proportional hazards was tested for each variable by adding a time-dependent covariate. All P values are 2-sided.
Results
Patient characteristics
Detailed demographic, hematologic, and transplantation-related factors of the 169 patients are shown in Table 1. Overall, 157 of 169 patients had one or more high-risk feature. The median time from diagnosis to transplantation was 6.5 months (range, 2.6-16.3 months). One-fourth of patients had a diagnostic WBC more than 100 × 109/L; and in another fourth, it exceeded 30 × 109/L. Thirty-seven of 99 patients with B lineage leukemia had WBC more than 30 × 109/L, and 10 of 32 patients with T-cell disease had a WBC more than 100 × 109/L. Forty-two percent took more than 8 weeks to remit, and 25% had high-risk cytogenetics. Approximately 40% of patients were older than 35 years.
Characteristics of patients 16 years of age or older who underwent URD SCT for Philadelphia-negative ALL in CR1, reported to the CIBMTR between 1995 and 2004
| Characteristics of patients . | No. evaluable . | N (%) . |
|---|---|---|
| No. of patients | 169 | |
| No. of centers | 85 | |
| Age, median, y (range) | 169 | 33 (16-59) |
| Age at transplantation, y | 169 | |
| 20 or younger | 37 (22) | |
| 21 to 30 | 35 (21) | |
| 31 to 40 | 49 (29) | |
| 41 to 50 | 29 (17) | |
| 51 to 60 | 19 (11) | |
| Male sex | 169 | 101 (60) |
| Karnofsky score prior to transplantation less than 90% | 161 | 41 (25) |
| Lineage | 169 | |
| T | 169 | 32 (19) |
| B | 99 (58) | |
| Unknown | 38 (23) | |
| FAB subtype | 169 | |
| ALL, NOS | 8 (5) | |
| ALL T-cell | 32 (19) | |
| ALL null cell (early Pre-B) | 14 (8) | |
| ALL cALLa (including Pre-B) | 58 (34) | |
| ALL, B-lineage, NOS | 27 (16) | |
| ALL other | 30 (18) | |
| WBC at diagnosis, ×109/L | 155 | 31 (<1-776) |
| Less than 30 | 77 (50) | |
| 30 to 100 | 40 (26) | |
| 100 to 200 | 25 (16) | |
| More than 200 | 13 (8) | |
| Site of extramedullary disease | 169 | |
| CNS with or without another | 21 (12) | |
| Other* | 10 (6) | |
| No extramedullary disease | 138 (82) | |
| Time from diagnosis to CR1 | 168 | |
| Less than 4 wk | 43 (26) | |
| 4 to 8 wk | 55 (33) | |
| More than 8 wk | 70 (42) | |
| Time from CR1 to transplantation | 169 | |
| Less than 3 mo | 58 (34) | |
| 3 to 6 mo | 70 (41) | |
| More than 6 mo | 41 (24) | |
| Time from diagnosis to transplantation | 169 | |
| Less than 3 mo | 4 (2) | |
| 3 to 6 mo | 67 (40) | |
| More than 6 mo | 98 (58) | |
| Cytogenetics | 169 | |
| t(4;11) with or without others | 27 (16) | |
| Hypodiploid (no t(4;11) or t(8,14)) with or without others | 10 (6) | |
| Five or more abnormalities | 5 (3) | |
| Other | 60 (35) | |
| No abnormalities | 67 (40) | |
| Conditioning regimen | ||
| Cy plus TBI with or without another | 129 (76) | |
| TBI with or without another (no Cy) | 25 (15) | |
| Cy with or without another (no TBI) | 11 (7) | |
| Other | 4 (2) | |
| TBI dose, cGy | 153 | 1200 (200-1500) |
| Less than 1300 | 31 (21) | |
| More than 1300 | 121 (79) | |
| Cyclophosphamide dose, mg/kg | 140 | 117 (8-200) |
| Less than 100 | 32 (23) | |
| 100 to 120 | 55 (39) | |
| More than 120 | 53 (38) | |
| Donor age, y | 163 | 33 (19-56) |
| 20 or younger | 2 (1) | |
| 21 to 30 | 62 (38) | |
| 31 to 40 | 66 (40) | |
| 41 to 50 | 31 (19) | |
| 51 to 60 | 2 (1) | |
| Donor-recipient sex match | 168 | |
| M-M | 75 (44) | |
| M-F | 32 (19) | |
| F-M | 25 (15) | |
| F-F | 36 (21) | |
| Donor-recipient CMV status | 166 | |
| +/+ | 41 (25) | |
| +/− | 28 (17) | |
| −/+ | 44 (27) | |
| −/− | 53 (31) | |
| Donor-recipient HLA match† | 169 | |
| Well matched | 70 (41) | |
| Partially matched | 70 (41) | |
| Mismatched | 29 (18) | |
| Graft type | 169 | |
| Bone marrow | 117 (69) | |
| Peripheral blood | 52 (31) | |
| Year of transplantation | 169 | |
| 1995 to 1996 | 27 (16) | |
| 1997 to 1998 | 23 (14) | |
| 1999 to 2000 | 27 (16) | |
| 2001 to 2002 | 36 (21) | |
| 2003 to 2004 | 56 (33) | |
| GVHD prophylaxis | 169 | |
| (FK506 or CsA) plus MTX with or without another | 130 (77) | |
| T-cell depletion‡ | 16 (9) | |
| Other§ | 23 (14) | |
| Median (range) follow-up of survivors, mo | 54 (3-133) |
| Characteristics of patients . | No. evaluable . | N (%) . |
|---|---|---|
| No. of patients | 169 | |
| No. of centers | 85 | |
| Age, median, y (range) | 169 | 33 (16-59) |
| Age at transplantation, y | 169 | |
| 20 or younger | 37 (22) | |
| 21 to 30 | 35 (21) | |
| 31 to 40 | 49 (29) | |
| 41 to 50 | 29 (17) | |
| 51 to 60 | 19 (11) | |
| Male sex | 169 | 101 (60) |
| Karnofsky score prior to transplantation less than 90% | 161 | 41 (25) |
| Lineage | 169 | |
| T | 169 | 32 (19) |
| B | 99 (58) | |
| Unknown | 38 (23) | |
| FAB subtype | 169 | |
| ALL, NOS | 8 (5) | |
| ALL T-cell | 32 (19) | |
| ALL null cell (early Pre-B) | 14 (8) | |
| ALL cALLa (including Pre-B) | 58 (34) | |
| ALL, B-lineage, NOS | 27 (16) | |
| ALL other | 30 (18) | |
| WBC at diagnosis, ×109/L | 155 | 31 (<1-776) |
| Less than 30 | 77 (50) | |
| 30 to 100 | 40 (26) | |
| 100 to 200 | 25 (16) | |
| More than 200 | 13 (8) | |
| Site of extramedullary disease | 169 | |
| CNS with or without another | 21 (12) | |
| Other* | 10 (6) | |
| No extramedullary disease | 138 (82) | |
| Time from diagnosis to CR1 | 168 | |
| Less than 4 wk | 43 (26) | |
| 4 to 8 wk | 55 (33) | |
| More than 8 wk | 70 (42) | |
| Time from CR1 to transplantation | 169 | |
| Less than 3 mo | 58 (34) | |
| 3 to 6 mo | 70 (41) | |
| More than 6 mo | 41 (24) | |
| Time from diagnosis to transplantation | 169 | |
| Less than 3 mo | 4 (2) | |
| 3 to 6 mo | 67 (40) | |
| More than 6 mo | 98 (58) | |
| Cytogenetics | 169 | |
| t(4;11) with or without others | 27 (16) | |
| Hypodiploid (no t(4;11) or t(8,14)) with or without others | 10 (6) | |
| Five or more abnormalities | 5 (3) | |
| Other | 60 (35) | |
| No abnormalities | 67 (40) | |
| Conditioning regimen | ||
| Cy plus TBI with or without another | 129 (76) | |
| TBI with or without another (no Cy) | 25 (15) | |
| Cy with or without another (no TBI) | 11 (7) | |
| Other | 4 (2) | |
| TBI dose, cGy | 153 | 1200 (200-1500) |
| Less than 1300 | 31 (21) | |
| More than 1300 | 121 (79) | |
| Cyclophosphamide dose, mg/kg | 140 | 117 (8-200) |
| Less than 100 | 32 (23) | |
| 100 to 120 | 55 (39) | |
| More than 120 | 53 (38) | |
| Donor age, y | 163 | 33 (19-56) |
| 20 or younger | 2 (1) | |
| 21 to 30 | 62 (38) | |
| 31 to 40 | 66 (40) | |
| 41 to 50 | 31 (19) | |
| 51 to 60 | 2 (1) | |
| Donor-recipient sex match | 168 | |
| M-M | 75 (44) | |
| M-F | 32 (19) | |
| F-M | 25 (15) | |
| F-F | 36 (21) | |
| Donor-recipient CMV status | 166 | |
| +/+ | 41 (25) | |
| +/− | 28 (17) | |
| −/+ | 44 (27) | |
| −/− | 53 (31) | |
| Donor-recipient HLA match† | 169 | |
| Well matched | 70 (41) | |
| Partially matched | 70 (41) | |
| Mismatched | 29 (18) | |
| Graft type | 169 | |
| Bone marrow | 117 (69) | |
| Peripheral blood | 52 (31) | |
| Year of transplantation | 169 | |
| 1995 to 1996 | 27 (16) | |
| 1997 to 1998 | 23 (14) | |
| 1999 to 2000 | 27 (16) | |
| 2001 to 2002 | 36 (21) | |
| 2003 to 2004 | 56 (33) | |
| GVHD prophylaxis | 169 | |
| (FK506 or CsA) plus MTX with or without another | 130 (77) | |
| T-cell depletion‡ | 16 (9) | |
| Other§ | 23 (14) | |
| Median (range) follow-up of survivors, mo | 54 (3-133) |
TBI indicates total body irradiation; CNS, central nervous system; CY, cyclophosphamide; CsA, cyclosporine; NOS, not otherwise specified; MTX, methotrexate; CR, complete remission; WBC, white blood cell; CMV, cytomegalovirus; GVHD, graft-versus-host disease; FK506, tacrolimus.
Other sites of extramedullary disease were pleura, skeleton (osteolysis), lymph nodes, spleen, liver, skin, lung, and kidney.
Donor-recipient HLA match was defined as follows:
Well matched: matched 8/8 at high-res HLA-A, -B, -C and -DRB1 (n = 41); matched 8/8 at high-res HLA-A, -B and -DRB1 and low-res at HLA-C (n = 5); matched 8/8 at low-res HLA-A, -B and -C and high-res at HLA-DRB1 (n = 21); matched 6/6 at high-res HLA-A, -B and -DRB1 (HLA-C unknown; n = 3).
Partially matched: Single allele MM (7/8) at high-res HLA-A, -B, -C and -DRB1 (n = 9); single antigen MM (7/8) at high-res HLA-A, -B, -C and -DRB1 (n = 15); single MM (7/8) at low-res HLA-A, -B and -C and high-res at HLA-DRB1 (n = 4); matched 8/8 at low-res HLA-A, -B and -C and -DRB1 (n = 4); single allele MM (5/6) at high-res HLA-A, -B and -DRB1 (HLA-C unknown; n = 2); matched 6/6 at low-res HLA-A and -B and high-res at HLA-DRB1 (HLA-C unknown) (n = 36).
Mismatched: 2 or more allele MM (<7/8) at high-res HLA-A, -B, -C and -DRB1 (n = 8); 2 or more MM with 1 antigen MM (<7/8) at high-res HLA-A, -B, -C and -DRB1 (n = 4); 2 or more MM with 2 or more antigen MM (<7/8) at high-res HLA-A, -B, -C and -DRB1 (n = 3); 2 or more MM (<7/8) at high-res HLA-A, -B and -DRB1 and low-res at HLA-C (n = 1); 2 or more MM (<7/8) at low-res HLA-A, -B and -C and high-res at HLA-DRB1 (n = 5); single MM (5/6) at low-res HLA-A and -B and high-res at HLA-DRB1 (HLA-C unknown; n = 6); matched (6/6) at low-res HLA-A and -B and -DRB1 (HLA-C unknown; n = 2).
Sixteen T-depleted grafts at 13 centers used: antibody and complement, 7; immunomagnetics, 4; soybean lectin, 1; elutriation, 1; and missing, 2.
Other GVHD prophylaxis were: FK506 + other (n = 10); CSA + other (n = 13).
Engraftment
Ninety-six percent of evaluable patients had myeloid engraftment, although 4% engrafted between days 28 and 100. Platelets more than 20 × 109/L at 28 days were achieved by 45%, and 73% achieved platelets more than 20 × 109/L at 100 days. None of the 37 patients with failed platelet engraftment survived, although 29 had hematopoietic recovery. Six patients did not have myeloid recovery; none survived. One of these patients received a second transplant.
Major outcomes
The major univariate outcomes of transplantation are shown in Table 2. One-year TRM occurred in slightly more than one-third of patients. Relapse occurred in 20%, which was expected given the risk status of the patients. Thirty-nine percent of patients survived 5 years. The results at 3 and 5 years were similar.
Outcomes after URD SCT for ALL in CR1: univariate analysis
| Outcome event . | N . | Probability (95% CI) . |
|---|---|---|
| ANC more than 0.5 × 109/L | 162 | |
| at 28 d | 92 (88-96) | |
| at 100 d | 96 (93-99) | |
| Acute GVHD, grades II-IV at 100 d | 167 | 50 (43-58) |
| Acute GVHD, grades III-IV at 100 d | 167 | 25 (19-32) |
| Chronic GVHD | 165 | |
| at 1 y | 38 (31-46) | |
| at 3 y | 43 (35-51) | |
| at 5 y | 43 (35-51) | |
| Treatment-related mortality | 166 | |
| at 100 d | 24 (18-31) | |
| at 1 y | 36 (29-43) | |
| at 3 y | 41 (33-49) | |
| at 5 y | 42 (34-50) | |
| Relapse | 166 | |
| at 1 y | 15 (10-21) | |
| at 3 y | 20 (14-26) | |
| at 5 y | 20 (14-26) | |
| Disease-free survival | 166 | |
| at 1 y | 49 (41-57) | |
| at 3 y | 39 (32-47) | |
| at 5 y | 38 (31-46) | |
| Overall survival | 169 | |
| at 1 y | 52 (45-60) | |
| at 3 y | 41 (33-49) | |
| at 5 y | 39 (31-47) |
| Outcome event . | N . | Probability (95% CI) . |
|---|---|---|
| ANC more than 0.5 × 109/L | 162 | |
| at 28 d | 92 (88-96) | |
| at 100 d | 96 (93-99) | |
| Acute GVHD, grades II-IV at 100 d | 167 | 50 (43-58) |
| Acute GVHD, grades III-IV at 100 d | 167 | 25 (19-32) |
| Chronic GVHD | 165 | |
| at 1 y | 38 (31-46) | |
| at 3 y | 43 (35-51) | |
| at 5 y | 43 (35-51) | |
| Treatment-related mortality | 166 | |
| at 100 d | 24 (18-31) | |
| at 1 y | 36 (29-43) | |
| at 3 y | 41 (33-49) | |
| at 5 y | 42 (34-50) | |
| Relapse | 166 | |
| at 1 y | 15 (10-21) | |
| at 3 y | 20 (14-26) | |
| at 5 y | 20 (14-26) | |
| Disease-free survival | 166 | |
| at 1 y | 49 (41-57) | |
| at 3 y | 39 (32-47) | |
| at 5 y | 38 (31-46) | |
| Overall survival | 169 | |
| at 1 y | 52 (45-60) | |
| at 3 y | 41 (33-49) | |
| at 5 y | 39 (31-47) |
Probabilities of ANC less than 0.5 × 109/L, acute GVHD, chronic GVHD, treatment-related mortality, and relapse were calculated using the cumulative incidence estimate. Disease-free survival and overall survival were calculated using the Kaplan-Meier product limit estimate.
GVHD indicates graft-versus-host disease.
Treatment-related mortality
A surprising finding was that 29 patients who were HLA-mismatched had a 83% higher TRM than the patients who were well or partially matched (who had similar TRMs, 40% and 41% at 5 years, respectively); this is shown in Figure 1. Five-year TRMs were similar in the less than 35-year-old and more than 35-year-old groups at 39% (range, 29%-50%) and 45% (range, 33%-58%), respectively (P = .48).
Cumulative incidence of TRM after unrelated donor transplantations for Philadelphia− ALL in first CR, by donor-recipient HLA-match.
Cumulative incidence of TRM after unrelated donor transplantations for Philadelphia− ALL in first CR, by donor-recipient HLA-match.
A surprising finding was that the 16 patients whose grafts were TCD had more than twice the TRM of the patients whose grafts were T-cell replete (Table 3). Thirteen of 16 patients receiving TCD grafts died of TRM (GVHD, 5; infection, 3; pulmonary toxicity, 2; and interstitial pneumonia, hemorrhage, and organ failure, one each). We examined this more fully by comparing characteristics of the 2 groups. Age and performance status were similar. The TCD group had a longer time from CR1 to transplantation. Conditioning and donor age were similar. Slightly more of the grafts were derived from marrow (88% vs 67%), and they occurred at an earlier time period (82% before 2001 vs 42%). The TCD group was slightly less well matched overall. A total of 19%, 44%, and 38% were well matched, partially matched, and mismatched, respectively, compared with 44%, 41%, and 15% in the T-replete group (P = .04). However, both TCD and HLA-mismatching were independently associated with TRM on multivariate analysis (Table 3).
Multivariate analysis of treatment-related mortality and relapse among patients 16 years of age and older who underwent URD transplants for Philadelphia-negative ALL in first complete remission
| Variable . | Event/no. evaluable . | Relative risk (95% CI) . | P . |
|---|---|---|---|
| Treatment-related mortality* | |||
| Donor-recipient HLA match | |||
| Well or partially matched† | 52/136 | 1.00§ | |
| Mismatched | 16/29 | 1.83 (1.04-3.22) | .037 |
| T-cell depletion | |||
| No | 56/149 | 1.00§ | |
| Yes | 12/16 | 2.67 (1.42-4.99) | .002 |
| Relapse | |||
| WBC at diagnosis | |||
| 100 × 109/L or less‡ | 22/113 | 1.00§ | Poverall‖ = .001 |
| More than 100 × 109/L | 10/38 | 2.96 (1.31-6.69) | P12 = .009 |
| Unknown | 5/14 | 5.40 (1.92-15.17) | P13 = .001 |
| Time from diagnosis to CR11 | |||
| 8 weeks or less | 15/97 | 1.00§ | |
| More than 8 weeks | 17/68 | 2.22 (1.09-4.53) | .028 |
| Donor-recipient CMV status | |||
| D−/R− | 30/54 | 1.00§ | |
| D+ or R+ | 70/111 | 2.34 (0.99-5.54) | .05 |
| Donor-recipient HLA match | |||
| Well or partially matched | 25/136 | 1.00§ | |
| Mismatched | 7/29 | 2.53 (1.05-6.08) | .038 |
| Variable . | Event/no. evaluable . | Relative risk (95% CI) . | P . |
|---|---|---|---|
| Treatment-related mortality* | |||
| Donor-recipient HLA match | |||
| Well or partially matched† | 52/136 | 1.00§ | |
| Mismatched | 16/29 | 1.83 (1.04-3.22) | .037 |
| T-cell depletion | |||
| No | 56/149 | 1.00§ | |
| Yes | 12/16 | 2.67 (1.42-4.99) | .002 |
| Relapse | |||
| WBC at diagnosis | |||
| 100 × 109/L or less‡ | 22/113 | 1.00§ | Poverall‖ = .001 |
| More than 100 × 109/L | 10/38 | 2.96 (1.31-6.69) | P12 = .009 |
| Unknown | 5/14 | 5.40 (1.92-15.17) | P13 = .001 |
| Time from diagnosis to CR11 | |||
| 8 weeks or less | 15/97 | 1.00§ | |
| More than 8 weeks | 17/68 | 2.22 (1.09-4.53) | .028 |
| Donor-recipient CMV status | |||
| D−/R− | 30/54 | 1.00§ | |
| D+ or R+ | 70/111 | 2.34 (0.99-5.54) | .05 |
| Donor-recipient HLA match | |||
| Well or partially matched | 25/136 | 1.00§ | |
| Mismatched | 7/29 | 2.53 (1.05-6.08) | .038 |
D+ or R+ CMV match vs D-/R-: RR = 1.36 (0.79-2.33); P = .27.
Partially matched vs well matched: RR = 1.02 (0.59-1.77); P = .94.
Thirty to 100 × 109/L vs less than 30 × 109/L: RR = 1.37 (0.52-3.61); P = .52.
Reference group.
Two degrees of freedom.
Relapse
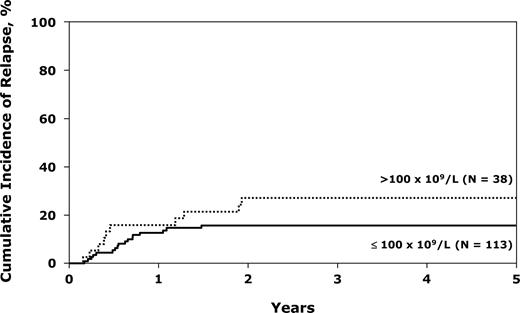
The only factor that significantly increased relapse was the diagnostic WBC (Table 3). A WBC more than 100 × 109/L more than doubled the risk (Figure 2). TCD was not associated with a higher chance of relapse, but this effect may be masked by the high TRM in the TCD group. Eighteen percent (95% CI, 10%-26%) of patients with adverse cytogenetics relapsed, not different from patients without this potential adverse factor. Five-year survival in the group with adverse cytogenetics (38%; 31%-46%) was also not different from the whole group of 169 patients.
Cumulative incidence of relapse after unrelated donor transplantations for Ph− ALL in first CR, by white blood cells.
Cumulative incidence of relapse after unrelated donor transplantations for Ph− ALL in first CR, by white blood cells.
The risk of relapse was not moderated by either higher-dose TBI or the development of GVHD. The relative risk (RR) of relapse in patients experiencing acute GVHD was 0.73 (95% CI, 0.36-1.49; P = .39). In patients with chronic GVHD, the RR was 0.47 (95% CI, 0.20-1.12; P = .09). Doses of TBI more than 13 Gy were associated with a RR of relapse of 0.68 (95% CI, 0.30-1.55; P = .36).
Survival
Cox regression analysis (Table 4) showed the following factors to be associated with death from any cause: diagnostic WBC more than 100 × 109/L (Figure 3A), HLA mismatch (Figure 3B), CMV seropositivity (donor or recipient positive, Figure 3C), a time to CR exceeding 8 weeks (Figure 3D), and TCD (Figure 3E). Diagnostic marrow biopsy to determine response may not have been performed in some patients until after 2 courses of chemotherapy; therefore, remission may not be documented within 8 weeks, thus limiting interpretation of this factor. Similar factors were associated with treatment failure (Table 5). Survival at 5 years was similar in the well-matched and partially matched groups (43% and 39%, respectively, P = not significant). Survival was somewhat higher in the less than 35-year-old group (43%, 95% CI 33%-54%) compared with the more than 35-year-old group (33%, 95% CI 23%-45%), but this was not statistically significant (P = .12). Performance status, GVHD, and time to achievement of CR did not significantly affect survival (data not shown).
Multivariate analysis of survival among patients 16 years of age and older who underwent URD transplants for Philadelphia-negative ALL in CR1
| Variable . | Event/no. evaluable . | Relative risk of death (95% CI) . | P . |
|---|---|---|---|
| WBC at diagnosis | |||
| 100 × 109/L or less* | 72/113 | 1.00§ | |
| More than 100 × 109/L | 25/38 | 1.73 (1.08-2.77) | P12 = .023 |
| Unknown | 11/14 | 2.32 (1.21-4.48) | P13 = .012 |
| Time from diagnosis to CR1 | |||
| 8 wk or less† | 52/97 | 1.00§ | |
| More than 8 wk | 45/68 | 1.74 (1.16-2.62) | .008 |
| Donor-recipient CMV status | |||
| D−/R− | 28/54 | 1.00§ | |
| D+ or R+ | 69/111 | 1.62 (1.02-2.56) | .040 |
| Donor-recipient HLA match | |||
| Well or partially matched‡ | 75/136 | 1.00§ | |
| Mismatched | 22/29 | 1.88 (1.16-3.05) | .010 |
| T-cell depletion | |||
| No | 84/149 | 1.00§ | |
| Yes | 13/16 | 2.62 (1.43-4.80) | .002 |
| Variable . | Event/no. evaluable . | Relative risk of death (95% CI) . | P . |
|---|---|---|---|
| WBC at diagnosis | |||
| 100 × 109/L or less* | 72/113 | 1.00§ | |
| More than 100 × 109/L | 25/38 | 1.73 (1.08-2.77) | P12 = .023 |
| Unknown | 11/14 | 2.32 (1.21-4.48) | P13 = .012 |
| Time from diagnosis to CR1 | |||
| 8 wk or less† | 52/97 | 1.00§ | |
| More than 8 wk | 45/68 | 1.74 (1.16-2.62) | .008 |
| Donor-recipient CMV status | |||
| D−/R− | 28/54 | 1.00§ | |
| D+ or R+ | 69/111 | 1.62 (1.02-2.56) | .040 |
| Donor-recipient HLA match | |||
| Well or partially matched‡ | 75/136 | 1.00§ | |
| Mismatched | 22/29 | 1.88 (1.16-3.05) | .010 |
| T-cell depletion | |||
| No | 84/149 | 1.00§ | |
| Yes | 13/16 | 2.62 (1.43-4.80) | .002 |
Thirty to 100 × 109/L versus less than 30 × 109/L: RR = 0.97 (0.56-1.68); P = .91.
Less than four weeks versus 4 to 8 weeks: RR = 0.73 (0.41-1.30); P = .28.
Partially matched versus well matched: RR = 1.03 (0.64-1.65); P = .90.
Reference group.
Probability of overall survival after unrelated donor transplantations for Ph− ALL in first CR. White blood cells at diagnosis (A), donor-recipient HLA-match (B), donor-recipient CMV status (C), time from diagnosis to complete remission (D), and GVHD prophylaxis (E).
Probability of overall survival after unrelated donor transplantations for Ph− ALL in first CR. White blood cells at diagnosis (A), donor-recipient HLA-match (B), donor-recipient CMV status (C), time from diagnosis to complete remission (D), and GVHD prophylaxis (E).
Multivariate analysis of treatment failure (relapse or death) among patients 16 years of age and older who underwent URD transplants for Philadelphia-negative ALL in CR1
| Variable . | Event/no. evaluable . | Relative risk of relapse or death (95% CI) . | P . |
|---|---|---|---|
| WBC at diagnosis | |||
| 100 × 109/L or less* | 74/113 | 1.00§ | Poverall‖ = .004 |
| More than 100 × 109/L | 26/38 | 1.80 (1.13-2.87) | P12 = .014 |
| Unknown | 11/14 | 2.56 (1.32-4.95) | P13 = .005 |
| Time from diagnosis to CR1 | |||
| 8 wk or less† | 53/97 | 1.00§ | |
| More than 8 wk | 47/68 | 1.77 (1.18-2.65) | .006 |
| Donor-recipient CMV status | |||
| D−/R− | 30/54 | 1.00§ | |
| D+ or R+ | 70/111 | 1.61 (1.02v2.54) | .040 |
| Donor-recipient HLA match | |||
| Well or partially matched‡ | 77/136 | 1.00§ | |
| Mismatched | 23/29 | 2.09 (1.30-3.37) | .003 |
| T-cell depletion | |||
| No | 87/149 | 1.00§ | |
| Yes | 13/16 | 2.50 (1.37-4.58) | .003 |
| Variable . | Event/no. evaluable . | Relative risk of relapse or death (95% CI) . | P . |
|---|---|---|---|
| WBC at diagnosis | |||
| 100 × 109/L or less* | 74/113 | 1.00§ | Poverall‖ = .004 |
| More than 100 × 109/L | 26/38 | 1.80 (1.13-2.87) | P12 = .014 |
| Unknown | 11/14 | 2.56 (1.32-4.95) | P13 = .005 |
| Time from diagnosis to CR1 | |||
| 8 wk or less† | 53/97 | 1.00§ | |
| More than 8 wk | 47/68 | 1.77 (1.18-2.65) | .006 |
| Donor-recipient CMV status | |||
| D−/R− | 30/54 | 1.00§ | |
| D+ or R+ | 70/111 | 1.61 (1.02v2.54) | .040 |
| Donor-recipient HLA match | |||
| Well or partially matched‡ | 77/136 | 1.00§ | |
| Mismatched | 23/29 | 2.09 (1.30-3.37) | .003 |
| T-cell depletion | |||
| No | 87/149 | 1.00§ | |
| Yes | 13/16 | 2.50 (1.37-4.58) | .003 |
Thirty to 100 × 109/L versus less than 30 × 109/L: RR = 0.85 (0.49-1.49); P = .58.
Less than four weeks versus 4 to 8 weeks: RR = 0.76 (0.43-1.34); P = .34.
Partially matched versus well matched: RR = 0.98 (0.61-1.57); P = .94.
Reference group.
Two degrees of freedom.
We then examined the effect of patients having multiple adverse risk factors (WBC > 100 × 109/L, HLA mismatch, CMV+, time to CR > 8 weeks) on survival. Twelve patients had no risk factors, 60 had one, 66 had 2, and 22 had 3. Only 5 patients had either 4 or 5 risk factors, insufficient for separate analysis. Five-year survival in the patients with 1, 2, and 3 risk factors was 59% (95% CI 45%-71%), 29% (95% CI 18%-42%), and 16% (95% CI 4%-35%), respectively.
Causes of death
Ninety-seven of 169 patients died. Just more than one-fourth of patients died of relapse (Table 6). GVHD and infection were the major transplantation-related causes of death, and there were no unexpected categories of causes of death seen. The precise cause of death in a transplantation patient with multiple failing organs may be difficult to determine, and these data should be interpreted with caution.
Causes of death
| . | N (%) . |
|---|---|
| No. of patients | 169 |
| No. of deaths | 97 |
| Relapse | 25 (26) |
| Graft failure | 3 (3) |
| Infection | 22 (23) |
| GVHD | 14 (15) |
| Interstitial pneumonia | 3 (3) |
| ARDS | 6 (6) |
| Organ failure | 8 (8) |
| Secondary malignancy | 2 (2) |
| Hemorrhage | 3 (3) |
| Vascular | 1 (1) |
| Cardiotoxicity | 3 (3) |
| Pulmonary toxicity | 2 (2) |
| Other | 5 (5) |
| . | N (%) . |
|---|---|
| No. of patients | 169 |
| No. of deaths | 97 |
| Relapse | 25 (26) |
| Graft failure | 3 (3) |
| Infection | 22 (23) |
| GVHD | 14 (15) |
| Interstitial pneumonia | 3 (3) |
| ARDS | 6 (6) |
| Organ failure | 8 (8) |
| Secondary malignancy | 2 (2) |
| Hemorrhage | 3 (3) |
| Vascular | 1 (1) |
| Cardiotoxicity | 3 (3) |
| Pulmonary toxicity | 2 (2) |
| Other | 5 (5) |
GVHD indicates graft-versus-host disease; and ARDS, adult respiratory distress syndrome.
Discussion
This study reports the largest series of adult patients who underwent URD SCT for ALL in CR1. These results, even in such a high-risk group of patients (60 of 169, 36%, had one poor prognosis risk factor and 97 of 169, 57%, had multiple risk factors), indicate a need for improvement. The major barrier to success was excess TRM, with more than one-third of patients dying of transplantation-related causes. The principal modifiable cause of this was the use of mismatched donors, compatible with the results of the large recent study of Lee et al,16 which reported a 30% to 35% increase in mortality with single mismatches at HLA-A, B, C or DRB1 and higher mortality in patients with mismatches at 2 HLA loci. In our series, only 29 transplantations were HLA-mismatched, but the patients' poor survival suggests that other strategies, such as cord blood transplantations, should be pursued instead. Of interest is that older patients did not do worse than younger patients. Therefore, this study provides evidence that URD SCT can be safely applied to older individuals with Ph− ALL.
The high TRM in TCD patients was an interesting and unexpected finding but is not corroborated by all series. The purpose of TCD is to decrease TRM by decreasing acute and chronic GVHD as shown in German and United Kingdom data with antithymocyte globulin (ATG) and alemtuzamab.9 Kiehl et al9 reported 94 patients; 87 received TCD grafts and only 15% had grades III and IV acute GVHD. Nevertheless, the TRM was still 31%.
Interpretation of these data requires asking what would have been the outcome of these patients if they had received chemotherapy and not SCT. The reported survival of patients receiving chemotherapy with a WBC more than 100 × 109/L is 21% at 5 years, and the 5-year overall survival of patients with t(4;11), low hypodiploidy/near triploidy or more than 5 abnormalities was 24%, 22%, and 28%, respectively, in the Moorman et al study.6 Patients more than 35 years of age had 26% 5-year survival in the MRC-ECOG study (S. Richards, personal communication, 2007). Delayed time to CR is an adverse factor in most studies but not in the recent United Kingdom/United States study.1 It is also reasonable to surmise that the survival of patients with multiple risk factors would be even lower. In all these subsets, our observed DFS was superior after URD SCT.
So do the results of this study justify this aggressive intervention in high-risk adults with ALL? It is unlikely that a randomized study will ever address this issue and that only extrapolation from single-arm and registry-based studies will help resolve this issue. The critical need to choose the best first-line therapy is well illustrated by the publication by Fielding et al17 of the outcome of 609 adults who relapsed with ALL and had only a 7% 5-year survival. Only approximately 25% of these patients received a transplant as salvage therapy. However, outcomes of relapse should improve with better relapse regimens, earlier identification of relapse, and more judicious use of alternative donors.
What can be done to reduce the chance of relapse? In the sibling donor setting, doses of TBI more than 13 Gy result in superior outcomes irrespective of whether etoposide or cyclophosphamide was used.18 Twenty-one percent of our patients in this study received less than 13 Gy, which was not associated with a significantly inferior outcome on multivariate analysis. Acute and chronic GVHD can limit relapse risk,19,20 but we could not confirm that effect in this study, although there was a trend toward chronic GVHD being associated with less relapse. It is of note that patients with adverse cytogenetics did not have a higher risk of relapse in this study. Similar findings after URD SCT for acute myeloid leukemia were recently reported.21
Two innovations will require comparison with full-intensity URD SCT. Some relatively small studies of reduced intensity conditioning SCT using various regimens demonstrate 2-year survival in approximately one-third of patients and TRM in 4% to 27%, including many patients with advanced disease.22-25 This approach deserves testing in older patients with high-risk ALL in CR1. The other potentially important development will be increasing use of unrelated cord blood cells. Rocha et al,26 on behalf of the European Blood and Marrow Transplantation, reported 36% 2-year survival in 73 adult patients with ALL, many with advanced disease. The University of Minnesota reported 12 patient with 67% DFS, which compared favorably with transplantations using other donors.27
Some cautions in interpretation of these retrospective registry data are important. To have an URD SCT, these patients had to survive and remain in remission for a median of 6.5 months and be considered well enough for an allograft. As in other retrospective series, these selection factors may improve the reported outcomes.
In conclusion, however, the use of URD in adults with ALL in CR1 merits further investigation. A recent French study indicates that 10 of 10 matched URDs have a similar outcome to matched sibling donors in this disease.28 The next United Kingdom/United States intergroup study will prospectively analyze this approach in high-risk patients, including those with detectable MRD after 2 phases of chemotherapy. These transplantations will be confined to patients with allele-matched URD and will use reduced intensity conditioning in the more than 35-year-old group. This report does not provide the evidence base necessary to recommend URD SCT in all high-risk adults with ALL, but it does provide sufficient evidence to enter patients on studies that prospectively evaluate the role of this intervention in those patients at highest risk of relapse.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Public Health Service grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; Office of Naval Research; Health Resources and Services Administration (DHHS); and grants from AABB; Aetna; American International Group; American Society for Blood and Marrow Transplantation; Amgen; anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Baxter International; Bayer HealthCare Pharmaceuticals; BioOne Corporation; Blood Center of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Bristol-Myers Squibb Company; Cangene Corporation; Celgene Corporation; CellGenix; Cerus Corporation; Cubist Pharmaceuticals; Cylex; CytoTherm; DOR BioPharma; Dynal Biotech, an Invitrogen Company; EKR Therapeutics; Enzon Pharmaceuticals; Gambro BCT; Gamida Cell; Genzyme Corporation; Gift of Life Bone Marrow Foundation; GlaxoSmithKline; Histogenetics; HKS Medical Information Systems; Hospira; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery; Merck & Company; Medical College of Wisconsin; MGI Pharma; Millennium Pharmaceuticals; Miller Pharmaceutical Group; Milliman USA; Miltenyi Biotec; MultiPlan; National Marrow Donor Program; Nature Publishing Group; Oncology Nursing Society; Osiris Therapeutics; Pall Life Sciences; PDL BioPharma; Pfizer; Pharmion Corporation; Roche Laboratories; Schering Plough Corporation; Society for Healthcare Epidemiology of America; StemCyte; StemSoft Software; SuperGen; Sysmex; Teva Pharmaceutical Industries; Marrow Foundation; THERAKOS; University of Colorado Cord Blood Bank; ViaCell; Vidacare Corporation; ViraCor Laboratories; ViroPharma; and Wellpoint. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
National Institutes of Health
Authorship
Contribution: D.I.M. and D.J.W. designed the study; W.S.P., W.H., and M.-J.Z. did the statistical analysis; D.I.M., W.S.P., and D.J.W. prepared the manuscript; all authors participated in interpretation of data and approval of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel J. Weisdorf, University of Minnesota Hospitals and Clinics, Mayo Mail Code 480, 420 Delaware Street SE, Minneapolis, MN 55455; e-mail: weisd001@umn.edu.