Abstract
Toxicity-reduced conditioning is being used for allogeneic stem cell transplantation in older and/or comorbid patients. We report on the treatment of 133 patients (median age: 55.6 years [23-73 years]) with acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS; n = 81), myeloproliferative syndromes (MPS; n = 20), and lymphoid malignancies (n = 32) using conditioning with FBM: fludarabine (5 × 30 mg/m2), 1,3-bis(2-chloroethyl)-1-nitrosourea (or carmustine, BCNU; 2 × 200 mg/m2), and melphalan (140 mg/m2). Patients 55 years or older received fludarabine with reduced BCNU (2 ×150 mg/m2) and melphalan (110 mg/m2). After engraftment, chimerism analyses revealed complete donor hematopoiesis in 95.7% of patients. With a median follow-up of 58.5 months, 3- and 5-year overall survival (OS) was 53.0% and 46.1%, event-free survival (EFS) was 46.4% and 41.9%. No significant differences in OS and EFS were evident considering disease status (early vs advanced), patient age (<55 vs≥55 years), or donor type (related vs unrelated) in univariate and multivariate analyses. The cumulative 5-year incidence of death due to relapse was 20.1%. Nonrelapse mortality (NRM) after 100 days and 1 year was 15.8% and 26.3%. Among patients with AML/MDS, advanced cases (n = 64, including 61 with active disease) showed an OS of 44.6% and 42.4% after 3 and 5 years, respectively. Therefore, FBM conditioning combines effective disease control with low NRM.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is an established curative therapy in many patients with hematologic malignancies. A critical element for successful application of allogeneic HCT remains the conditioning regimen because it needs to provide sufficient myelosuppression for eradicating the underlying malignant disease together with effective immunosuppression of the host to ensure engraftment. However, toxicity caused by the conditioning regimen results in significant treatment-related morbidity and mortality
A heterogeneity of conditioning protocols were introduced in the past years, with substantial confusion regarding dose intensity, degree of myelosuppression and toxicity. Regarding dose intensity and correlated toxicity, all established protocols can be placed in a spectrum of conditioning regimens starting from minimal to reduced and conventional high dose intensity.1,2
Although older patients experience high incidences of various hematologic malignancies,3-5 regimen-related toxicity of protocols with conventional high dose intensity excluded these patients or patients with comorbidities from allogeneic HCT. Therefore fludarabine-based reduced intensity conditioning protocols have been developed to offer the potential curative treatment option with less toxicity to these patient populations. Studies from several groups using reduced intensity/toxicity conditioning in the treatment of myeloid malignancies have been encouraging.6-8 However, many published results included either small patient numbers or a heterogeneity of applied conditioning regimens with reduced toxicity and dose intensity.
Another challenge in this setting remains the treatment of active or advanced hematologic malignant diseases with allogeneic HCT. While minimal intensive conditioning protocols showed sufficient immunosuppression for engraftment combined with very little toxicity, control of advanced disease proved to be problematic. One explanation could be that in these approaches activity against the malignancies relied mainly on the graft-versus-leukemia (GVL)–effect and less on the cytotoxicity of the substances used for conditioning.9 Therefore more recent trials addressing active or advanced disease focused on protocols with reduced toxicity and increased dose intensity.10
Previously we established a regimen with reduced toxicity conditioning consisting of fludarabine in combination with the alkylating agents BCNU [1,3-bis(2-chloroethyl)-1-nitrosourea, or carmustine] and melphalan (FBM).11 The rationale was to enable engraftment of stem cells from related and unrelated donors and to apply moderate doses of cytotoxic agents with broad activity, good CNS penetration but negligible heart, lung, bladder, renal, or gastrointestinal toxicity.
Conditioning with FBM resulted in marrow aplasia and complete chimerism with little nonhematopoietic tissue toxicity.11 The regimen was successfully used in 19 patients 60 years of age or older with myeloid leukemias who underwent related and unrelated donor transplantation6 and in 34 patients 60 years of age or older with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) who received transplants from unrelated donors.12
Based on this encouraging result we now report long-term follow-up results of 133 consecutive patients with hematologic malignancies included in a 2-center, prospective, nonrandomized, open-label phase 2 clinical trial in adult patients with hematologic and solid tumors.
Methods
Patient characteristics and eligibility criteria
Between August 1998 and November 2003, 153 consecutive adult patients in Freiburg and Regensburg were enrolled in the study. The included patients presented with hematologic and nonhematologic malignancies treatable by allogeneic HCT but were ineligible for conventional intensity conditioning. Therefore, 112 patients were included because of age 50 years or older. The remaining 41 patients were treated with the toxicity-reduced regimen because of the decision of the responsible physician. Overlapping reasons for these subjective decisions were poor performance status and/or concomitant disease and/or preceding extensive therapies such as failed autologous HCT. Exclusion criteria were active central nervous system disease, pregnancy, infection with human immunodeficiency virus, active hepatitis B or C, severe impairment of renal, cardiac or hepatic function, and Karnofsky performance score less than 60%.
The diagnoses included AML (n = 60), MDS (n = 23), acute lymphoblastic leukemia (ALL; n = 4), chronic myeloid leukemia (CML; n = 11), non-Hodgkin lymphoma (NHL; n = 19), chronic lymphocytic leukemia (CLL; n = 8), multiple myeloma (MM; n = 14), myeloproliferative syndromes (MPS; n = 9), melanoma (n = 3), and renal cell carcinoma (n = 2). Nine patients had known active fungal infection before transplantation. For statistical purposes regarding treatment-related toxicity and outcome, patients with nonhematologic malignancies (renal carcinoma, n = 2, and melanoma, n = 3) as well as 13 patients with prior autologous or allogeneic HCT diagnosed with NHL (6 patients), MM (3 patients), CLL (1 patient), AML (2 patients), and ALL (1 patient) were excluded from further analysis. For overall analysis of graft-versus-host disease (GVHD) prophylaxis, the 2 patients treated with cyclosporine A (CsA) only (both with MM) were also excluded. The characteristics of the study patients (n = 133) are presented in Table 1.
Characteristics of patients and grafts
| Parameter . | Total, n = 133 . |
|---|---|
| Patients | |
| Male | 74 (55.6) |
| Female | 59 (44.4) |
| Median age plus or minus SD, y (range) | 55.6 ± 9.4 (23.8-73.7) |
| Diagnoses | |
| CML | 11 (8.3) |
| CP1 | 8 |
| AP/BC | 3 |
| ALL | 3 (2.3) |
| CR2 | 1 |
| Relapse/refractory | 2 |
| MDS | 23 (17.3) |
| RA | 8 |
| RARS | 1 |
| RAEB/t, CMMoL (untreated) | 14 |
| AML | 29 (21.8) |
| CR1 | 6 |
| Relapse/primary refractory | 19 |
| CR2 or greater | 3 |
| Untreated | 1 |
| sAML | 29 (21.8) |
| CR1 | 2 |
| Relapse/refractory | 16 |
| Untreated | 11 |
| MPS/OMF | 9 (6.8) |
| Untreated/CP1 | 4 |
| CP2 | 1 |
| BC | 4 |
| Myeloma, all relapse/refractory/PR | 9 (6.8) |
| Lymphoma | 13 (9.8) |
| CR1 | 1 |
| CR2 or greater | 1 |
| Relapse/refractory/PR | 11 |
| CLL, all refractory | 7 (5.3%) |
| Remission status | |
| Total | 133 |
| Early | 27 (20.3) |
| Advanced | 106 (79.7) |
| AML/MDS | 81 |
| Early | 17 (21.0) |
| Advanced | 64 (79.0) |
| Donor type | 127 |
| Age, y (range) | 44.4 (20-74) |
| Related, fully matched | *68 (51.1) |
| Unrelated, fully matched | †59 (44.4) |
| Unrelated, minor HLA-mismatched | 6 (4.5) |
| Donor sex match | |
| Patient male/donor female | 31 (23.3) |
| Patient female/donor male | 33 (24.8) |
| Match | 69 (51.9) |
| CMV status | |
| Patient neg/donor neg | 23 (17.3) |
| Patient neg/donor pos | 18 (13.5) |
| Patient pos/donor neg | 36 (27.1) |
| Patient pos/donor pos | 45 (33.8) |
| NE | 1 (0.8) |
| GVHD prophylaxis | |
| CsA plus mMtx | ‡23 (17.3) |
| CsA plus MMF | §¶108 (81.2) |
| Graft | |
| Source | |
| Bone marrow | 15 (11.3) |
| Peripheral blood | 116 (87.2) |
| Both | 2 (1.5) |
| Values | |
| Bone marrow | |
| WBC, × 108 cells/kg (15 pt) | 3.7 (1.9-14.0) |
| CD34, × 106 cells/kg (13 pt) | 3.0 (1.0-9.6) |
| CD3, × 108 cells/kg (11 pt) | 0.5 (0.2-2.4) |
| Peripheral blood | |
| WBC, × 108 cells/kg (114 pt) | 13.5 (4.2-32.7) |
| CD34, × 106 cells/kg (116 pt) | 6.7 (1.7-14.0) |
| CD3, × 108 cells/kg (108 pt) | 2.8 (1.1-6.6) |
| Both | |
| WBC, × 108 cells/kg (2 pt) | 35.2 |
| CD34, × 06 cells/kg (2 pt) | 2.5 |
| CD3, × 108 cells/kg (2 pt) | 3.3 |
| Parameter . | Total, n = 133 . |
|---|---|
| Patients | |
| Male | 74 (55.6) |
| Female | 59 (44.4) |
| Median age plus or minus SD, y (range) | 55.6 ± 9.4 (23.8-73.7) |
| Diagnoses | |
| CML | 11 (8.3) |
| CP1 | 8 |
| AP/BC | 3 |
| ALL | 3 (2.3) |
| CR2 | 1 |
| Relapse/refractory | 2 |
| MDS | 23 (17.3) |
| RA | 8 |
| RARS | 1 |
| RAEB/t, CMMoL (untreated) | 14 |
| AML | 29 (21.8) |
| CR1 | 6 |
| Relapse/primary refractory | 19 |
| CR2 or greater | 3 |
| Untreated | 1 |
| sAML | 29 (21.8) |
| CR1 | 2 |
| Relapse/refractory | 16 |
| Untreated | 11 |
| MPS/OMF | 9 (6.8) |
| Untreated/CP1 | 4 |
| CP2 | 1 |
| BC | 4 |
| Myeloma, all relapse/refractory/PR | 9 (6.8) |
| Lymphoma | 13 (9.8) |
| CR1 | 1 |
| CR2 or greater | 1 |
| Relapse/refractory/PR | 11 |
| CLL, all refractory | 7 (5.3%) |
| Remission status | |
| Total | 133 |
| Early | 27 (20.3) |
| Advanced | 106 (79.7) |
| AML/MDS | 81 |
| Early | 17 (21.0) |
| Advanced | 64 (79.0) |
| Donor type | 127 |
| Age, y (range) | 44.4 (20-74) |
| Related, fully matched | *68 (51.1) |
| Unrelated, fully matched | †59 (44.4) |
| Unrelated, minor HLA-mismatched | 6 (4.5) |
| Donor sex match | |
| Patient male/donor female | 31 (23.3) |
| Patient female/donor male | 33 (24.8) |
| Match | 69 (51.9) |
| CMV status | |
| Patient neg/donor neg | 23 (17.3) |
| Patient neg/donor pos | 18 (13.5) |
| Patient pos/donor neg | 36 (27.1) |
| Patient pos/donor pos | 45 (33.8) |
| NE | 1 (0.8) |
| GVHD prophylaxis | |
| CsA plus mMtx | ‡23 (17.3) |
| CsA plus MMF | §¶108 (81.2) |
| Graft | |
| Source | |
| Bone marrow | 15 (11.3) |
| Peripheral blood | 116 (87.2) |
| Both | 2 (1.5) |
| Values | |
| Bone marrow | |
| WBC, × 108 cells/kg (15 pt) | 3.7 (1.9-14.0) |
| CD34, × 106 cells/kg (13 pt) | 3.0 (1.0-9.6) |
| CD3, × 108 cells/kg (11 pt) | 0.5 (0.2-2.4) |
| Peripheral blood | |
| WBC, × 108 cells/kg (114 pt) | 13.5 (4.2-32.7) |
| CD34, × 106 cells/kg (116 pt) | 6.7 (1.7-14.0) |
| CD3, × 108 cells/kg (108 pt) | 2.8 (1.1-6.6) |
| Both | |
| WBC, × 108 cells/kg (2 pt) | 35.2 |
| CD34, × 06 cells/kg (2 pt) | 2.5 |
| CD3, × 108 cells/kg (2 pt) | 3.3 |
Totals are numbers (%) except where defined otherwise. Graft cell values are given per kilogram of patient body weight.
CP indicates chronic phase; AP/BC, acute phase/blast crisis; CR, complete remission; RA, refractory anemia; RARS, refractory anemia with ringed sideroblasts; RAEB/t, refractory anemia with excess blasts in transformation; CMMoL, chronic myelomonocytic leukemia; PR, partial remission; sAML, secondary AML; OMF, osteomyelofibrosis; neg, negative; pos, positive; NE, not evaluable; MMF, mycophenolate mofetil; WBC, white blood cells; and pt, patients.
ATG was used for conditioning in 1 patient.
Three patients did not receive ATG conditioning although ATG was used for conditioning in all unrelated donors.
Including 3 patients with additional ATG.
Including 60 patients with additional ATG.
Including 2 patients with additional prednisolone.
HLA typing and stem cell graft
Patients and their related or unrelated donors were matched for HLA-A and HLA-B class I loci by serologic (2-digit) and for DRB1 and DQB1 class II alleles by high-resolution DNA-typing (4-digit) techniques. Patients received granulocyte colony-stimulating factor (G-CSF)–mobilized, unmanipulated peripheral blood stem cells (n = 116), bone marrow (n = 15), or both (n = 2) from matched related (n = 68), matched unrelated (n = 52), or minor HLA-mismatched unrelated (n = 6) donors. Eight CML patients in chronic phase 1 (CP1) received bone marrow purposely. Further donor and stem cell graft characteristics are shown in Table 1.
Transplantation procedure, conditioning, GVHD prophylaxis
As preparative conditioning chemotherapy before allogeneic HCT all patients received the toxicity-reduced FBM regimen as described previously.11 For patients younger than 55 years the protocol included fludarabine 5 × 30 mg/m2 (days −9 to −5), BCNU 2 × 200 mg/m2 (days −7 to −6), and melphalan 140 mg/m2 on day −4. A dose-modified regimen (fludarabine 5 × 30 mg/m2 from days −9 to −5, BCNU 2 × 150 mg/m2 from days −7 to −6, and melphalan 110 mg/m2 on day −4) was used for patients 55 years or older. Three patients younger than 55 years received doses given to older patients because of heavy pretreatment or reduced performance status.
GVHD prophylaxis for related and unrelated donors consisted of CsA starting at day −3 at a dose of 2.5 mg/kg twice a day, with an initial through level of 250 to 350 ng/mL, and mMTX (5 mg/m2 at days +1, +3, +6) in 23 patients. Due to toxicity problems, mMTX was replaced by mycophenolate mofetil (MMF) in an amendment of the study protocol, and therefore the following 108 patients received CsA and additional MMF (2 g daily starting day −1). MMF was tapered from day 30 and CsA was tapered from day 80 to day 100 after transplantation in the absence of acute GVHD. In addition, all patients with unrelated donors received rabbit anti–T-lymphocyte globulin (ATG-F; Fresenius, Graefelfing, Germany) at a total dose of 20 to 60 mg/kg at days −2 and −1. Among these, 1 patient with severe thrombopenia due to platelet antibodies and 2 patients with infectious complications before transplantation did not receive ATG-F. Two patients with CML in CP1 and matched related grafts received prednisolone in addition to CsA and MMF.
Transfusion of the stem cell graft, supportive care, antimicrobial prophylaxis and treatment, and transfusion of blood products were performed according to standard good clinical practice (GCP) procedures.
Evaluations
Chimerism analysis and quantification were performed on marrow and/or peripheral blood samples before transplantation and on days +30, +100, +180, and +360 by fluorescence in situ hypridization or variable number of tandem repeats studies as described earlier.11 Mixed chimerism was defined as the presence of more than 5% host-derived cells on more than one occasion in any of the whole blood or the bone marrow samples. Treatment-related toxicity was assessed using the criteria of Bearman et al.13
Statistical analyses
Data were analyzed as of December 31, 2005. The Kaplan-Meier method was used to estimate overall (OS) and event-free (EFS) survival probabilities with accompanying 95% confidence intervals (CIs). OS was calculated as time from transplantation until death from any cause. EFS was determined as time from transplantation until relapse or death from any cause. Patients who have died without evidence of relapse or progression were considered as cases of nonrelapse mortality (NRM). Rates of death due to relapse and nonrelapse mortality were estimated as cumulative incidence rates, where relapse and NRM were considered as competing risks. Acute and chronic GVHD were analyzed as cumulative incidence rates with death of any cause as competing risk.
The analysis of potential prognostic factors for OS and EFS of the total patients cohort and the subgroup of AML/MDS patients was performed with Cox proportional hazards regression models. Univariate and multivariate models with a backward elimination strategy for variable selection included diagnosis, disease stage at BMT, patient age and sex, donor choice, age and sex, HLA mismatch, graft choice, GVHD prophylaxis, and time of BMT after diagnosis. The impact of these factors on relapse mortality and nonrelapse mortality was investigated in the same manner as for OS/EFS by evaluating cause specific hazard ratios with Cox models.
Similarly, disease stage at BMT, patient age and sex, donor choice and sex, HLA mismatch, GVHD prophylaxis, and graft choice were considered as possible prognostic factors for acute grade II-IV GVHD and extensive chronic GVHD. For the analysis of acute GVHD, observations were censored at day 101. Cause-specific hazards were analyzed by univariate and multivariate Cox models. P values reported here refer to the Cox regression models described, and P values less than .05 were considered significant. Calculations were performed using SAS software, version 9 (SAS Institute, Cary, NC).
Study conduct
The study protocol and an amendment regarding the GVHD prophylaxis for matched and mismatched unrelated donor transplantation were approved by the local Internal Review Board and the Ethics Committee of both participating transplant centers (Albert-Ludwigs University Medical Center and University of Regensburg Medical Center). The study was carried out according to the Helsinki Declaration. Written informed consent was obtained from all patients and donors in accordance with the Declaration of Helsinki after they were informed about the investigational nature of the study.
Results
Patient characteristics
A total of 133 patients who received transplants in Freiburg and Regensburg were analyzed in this study. The cohort included patients who have been reported earlier,6,11,12 with an extended follow-up analysis. Characteristics of patients, donors, and grafts are shown in Table 1. The median patient age at allogeneic HCT was 55.6 years (range from 23.8 to 73.7 years). The patients presented with several hematologic malignancies which are listed in more detail in Table 1. Among the latter, a total of 81 patients with myeloid diseases (MDS/sAML/AML) were enrolled in the study.
In the whole population the majority of patients (n = 106) had advanced stages of the underlying disease at the time of allogeneic HCT. Advanced disease was defined as either primary refractory, relapsing, greater than or equal to CR2/CP2, or untreated. Only 27 patients presented with early disease defined as CR1/CP1 or MDS RA/RARS. In the subgroup of myeloid malignancies (AML/MDS) a total of 79.0% (64/81 patients) received transplants in an advanced disease stage, of whom almost all (61 patients) had active disease defined as untreated, primary refractory, or untreated relapsed. Among the latter, 33 patients showed blasts in the peripheral blood (median, 21.1%; range, 1%-98%) and in the marrow (median, 51,9%; range, 8%-98%) at the time of HCT. Siblings (68 cases) and unrelated donors (65 cases) were almost equally used as stem cell donors. In the overwhelming majority of cases (87.2%) G-CSF mobilized PBSC was used for allografting. Most patients received GVHD prophylaxis consisting of CsA/MMF (n = 108) and additional ATG in the case of unrelated donors.
Engraftment and chimerism
Engraftment of allogeneic stem cells could be documented in 130 patients demonstrating sufficient immunosuppressive capacity of the conditioning protocol. Two patients died by sepsis and pneumonia on day +3 or +13 before engraftment could occur. Only one fatal graft failure occurred in a patient with blast crisis in osteomyelofibrosis. Median time for reconstitution of myelopoiesis (WBC > 109/L [1000/μL]) was 12.7 days (range, 1-41). One patient did not experience leukopenia (WBC < 109/L [1000/μL]) at any time. Platelet counts more than 20 × 109/L (20 000/μL) were reached at a median of 17.4 days (range, 5-113).
Chimerism analyses could be evaluated in 92 patients with 88 cases (95.7%) of complete donor chimerism after 30 days. Therefore at least in the reported cases the high degree of donor hematopoiesis after reconstitution reflects almost complete host myelosuppression by the conditioning regimen.
Toxicity, GVHD, disease control and nonrelapse mortality
Considering 130 patients with hematopoietic recovery after FBM conditioning, 112 patients (86.2%) showed new or sustained complete remission of their underlying disease. Ten additional patients experienced partial responses. The relapse related and the nonrelapse mortalities (NRM) have been analyzed as competing risks. In total, 25 patients died by progression of the underlying disease. The entire cohort showed cumulative 1-, 3-, and 5-year relapse rates of 12.0%, 16.7%, and 20.1%, respectively (Figure 1A, Table 2). Among those, patients with early disease experienced a relapse mortality of 3.7% after 1 year with no additional cases later on (Figure 1B). Regarding the stem cell donor, in cases with related donors 1-, 3-, and 5-year relapse rates were 10.3%, 11.8%, and 15.7% and with unrelated donors 13.8%, 22.0%, and 24.4%, respectively (Table 2). The differences in relapse rate between the used donors did not reach statistical significance (P = .12).
Relapse, non relapse mortality, and GVHD of entire patient cohort. Total patient cohort (n = 133): (A) Cumulative incidence of relapse and nonrelapse mortality (NRM). (B) Cumulative incidence of relapse according to disease status at time of HCT (advanced versus early). (C) Cumulative incidence of acute GVHD grades II, III, and IV. (D) Cumulative incidence of extensive chronic GVHD.
Relapse, non relapse mortality, and GVHD of entire patient cohort. Total patient cohort (n = 133): (A) Cumulative incidence of relapse and nonrelapse mortality (NRM). (B) Cumulative incidence of relapse according to disease status at time of HCT (advanced versus early). (C) Cumulative incidence of acute GVHD grades II, III, and IV. (D) Cumulative incidence of extensive chronic GVHD.
Outcome of entire patient cohort (n = 133)
| . | Day 100 . | 1 year . | 3 years . | 5 years . | P . |
|---|---|---|---|---|---|
| Overall survival | 82.7 (76.3-89.1) | 61.7 (53.4-69.9) | 53.0 (44.5-61.6) | 46.1 (36.9-55.2) | |
| Disease stage at HSCT | |||||
| Early | 88.9 (77.0-100.7) | 63.0 (44.7-81.2) | 63.0 (44.7-81.2) | 57.7 (38.3-77.1) | .30* |
| Advanced | 81.1 (73.7-88.6) | 61.3 (52.0-70.6) | 50.4 (40.8-60.1) | 43.0 (32.8-53.2) | |
| Patient age | |||||
| Younger than 55 y | 84.0 (73.8-94.2) | 66.0 (52.9-79.1) | 60.0 (46.4-73.6) | 54.8 (40.6-69.0) | .29* |
| 55 y or older | 81.9 (73.6-90.2) | 59.0 (48.5-69.6) | 48.9 (38.1-59.8) | 40.8 (29.1-52.5) | |
| Donor | |||||
| Related | 82.4 (73.3-91.4) | 64.7 (53.3-76.1) | 58.7 (47.0-70.4) | 48.9 (36.3-61.5) | .37* |
| Unrelated | 83.1 (74.0-92.2) | 58.5 (46.5-70.4) | 47.0 (34.6-59.3) | 44.6 (32.1-57.1) | |
| Donor sex | |||||
| Female | 86.0 (76.9-95.0) | 70.2 (58.3-82.1) | 64.6 (52.2-77.1) | 56.8 (43.0-70.6) | .07* |
| Male | 80.3 (71.3-89.2) | 55.3 (44.1-66.4) | 44.4 (33.1-55.6) | 38.4 (26.8-50.0) | |
| Event free survival | 79.7 (72.9-86.5) | 56.4 (48.0-64.8) | 46.4 (37.9-54.9) | 41.9 (33.1-50.7) | |
| Disease stage at HSCT | |||||
| Early | 88.9 (77.0-100.7) | 59.3 (40.7-77.8) | 55.6 (36.8-74.3) | 50.5 (31.0-70.0) | .35* |
| Advanced | 77.4 (69.4-85.3) | 55.7 (46.2-65.1) | 44.0 (34.5-53.5) | 39.6 (29.8-49.4) | |
| Patient age | |||||
| Younger than 55 y | 78.0 (66.5-89.5) | 64.0 (50.7-77.3) | 56.0 (42.2-69.8) | 48.2 (33.8-62.6) | .20* |
| 55 y or older | 80.7 (72.2-89.2) | 51.8 (41.1-62.6) | 40.6 (30.0-51.3) | 38.4 (27.4-49.3) | |
| Donor | |||||
| Related | 82.4 (73.3-91.4) | 64.7 (53.3-76.1) | 54.3 (42.4-66.2) | 46.5 (34.1-59.0) | .14* |
| Unrelated | 76.9 (66.7-87.2) | 47.7 (35.5-59.8) | 38.1 (26.2-50.0) | 38.1 (26.2-50.0) | |
| Donor sex | |||||
| Female | 86.0 (76.9-95.0) | 68.4 (56.4-80.5) | 59.2 (46.3-72.1) | 51.7 (37.9-65.5) | .04** |
| Male | 75.0 (65.3-84.7) | 47.4 (36.1-58.6) | 36.8 (25.9-47.6) | 34.6 (23.6-45.6) | |
| Relapse | 1.5 (0.4-6.0) | 12.0 (7.6-19.0) | 16.7 (11.4-24.5) | 20.1 (14.0-28.7) | |
| Disease stage at HSCT | |||||
| Early | 0.0 | 3.7 (0.5-25.3) | 3.7 (0.5-25.3) | 3.7 (0.5-25.3) | .07* |
| Advanced | 1.9 (0.5-7.4) | 14.2 (8.9-22.6) | 20.1 (13.7-29.4) | 24.3 (17.0-34.7) | |
| Donor | |||||
| Related | 2.9 (0.8-11.5) | 10.3 (5.1-20.8) | 11.8 (6.1%-22.6) | 15.7 (8.8-27.9) | .13* |
| Unrelated | 0.0 | 13.8 (7.6-25.4) | 22.0 (13.8%-35.0) | 24.4 (15.6-38.1) | |
| NRM | 15.8 (10.7-23.4) | 26.3 (19.8-35.0) | 30.3 (23.3-39.2) | 33.9 (26.4-43.5) |
| . | Day 100 . | 1 year . | 3 years . | 5 years . | P . |
|---|---|---|---|---|---|
| Overall survival | 82.7 (76.3-89.1) | 61.7 (53.4-69.9) | 53.0 (44.5-61.6) | 46.1 (36.9-55.2) | |
| Disease stage at HSCT | |||||
| Early | 88.9 (77.0-100.7) | 63.0 (44.7-81.2) | 63.0 (44.7-81.2) | 57.7 (38.3-77.1) | .30* |
| Advanced | 81.1 (73.7-88.6) | 61.3 (52.0-70.6) | 50.4 (40.8-60.1) | 43.0 (32.8-53.2) | |
| Patient age | |||||
| Younger than 55 y | 84.0 (73.8-94.2) | 66.0 (52.9-79.1) | 60.0 (46.4-73.6) | 54.8 (40.6-69.0) | .29* |
| 55 y or older | 81.9 (73.6-90.2) | 59.0 (48.5-69.6) | 48.9 (38.1-59.8) | 40.8 (29.1-52.5) | |
| Donor | |||||
| Related | 82.4 (73.3-91.4) | 64.7 (53.3-76.1) | 58.7 (47.0-70.4) | 48.9 (36.3-61.5) | .37* |
| Unrelated | 83.1 (74.0-92.2) | 58.5 (46.5-70.4) | 47.0 (34.6-59.3) | 44.6 (32.1-57.1) | |
| Donor sex | |||||
| Female | 86.0 (76.9-95.0) | 70.2 (58.3-82.1) | 64.6 (52.2-77.1) | 56.8 (43.0-70.6) | .07* |
| Male | 80.3 (71.3-89.2) | 55.3 (44.1-66.4) | 44.4 (33.1-55.6) | 38.4 (26.8-50.0) | |
| Event free survival | 79.7 (72.9-86.5) | 56.4 (48.0-64.8) | 46.4 (37.9-54.9) | 41.9 (33.1-50.7) | |
| Disease stage at HSCT | |||||
| Early | 88.9 (77.0-100.7) | 59.3 (40.7-77.8) | 55.6 (36.8-74.3) | 50.5 (31.0-70.0) | .35* |
| Advanced | 77.4 (69.4-85.3) | 55.7 (46.2-65.1) | 44.0 (34.5-53.5) | 39.6 (29.8-49.4) | |
| Patient age | |||||
| Younger than 55 y | 78.0 (66.5-89.5) | 64.0 (50.7-77.3) | 56.0 (42.2-69.8) | 48.2 (33.8-62.6) | .20* |
| 55 y or older | 80.7 (72.2-89.2) | 51.8 (41.1-62.6) | 40.6 (30.0-51.3) | 38.4 (27.4-49.3) | |
| Donor | |||||
| Related | 82.4 (73.3-91.4) | 64.7 (53.3-76.1) | 54.3 (42.4-66.2) | 46.5 (34.1-59.0) | .14* |
| Unrelated | 76.9 (66.7-87.2) | 47.7 (35.5-59.8) | 38.1 (26.2-50.0) | 38.1 (26.2-50.0) | |
| Donor sex | |||||
| Female | 86.0 (76.9-95.0) | 68.4 (56.4-80.5) | 59.2 (46.3-72.1) | 51.7 (37.9-65.5) | .04** |
| Male | 75.0 (65.3-84.7) | 47.4 (36.1-58.6) | 36.8 (25.9-47.6) | 34.6 (23.6-45.6) | |
| Relapse | 1.5 (0.4-6.0) | 12.0 (7.6-19.0) | 16.7 (11.4-24.5) | 20.1 (14.0-28.7) | |
| Disease stage at HSCT | |||||
| Early | 0.0 | 3.7 (0.5-25.3) | 3.7 (0.5-25.3) | 3.7 (0.5-25.3) | .07* |
| Advanced | 1.9 (0.5-7.4) | 14.2 (8.9-22.6) | 20.1 (13.7-29.4) | 24.3 (17.0-34.7) | |
| Donor | |||||
| Related | 2.9 (0.8-11.5) | 10.3 (5.1-20.8) | 11.8 (6.1%-22.6) | 15.7 (8.8-27.9) | .13* |
| Unrelated | 0.0 | 13.8 (7.6-25.4) | 22.0 (13.8%-35.0) | 24.4 (15.6-38.1) | |
| NRM | 15.8 (10.7-23.4) | 26.3 (19.8-35.0) | 30.3 (23.3-39.2) | 33.9 (26.4-43.5) |
Data are percentages (ranges) of patients.
CI indicates 95% confidence interval.
Univariate analysis (Cox proportional hazards regression models).
Multivariate analysis (Cox proportional hazards regression models).
The respective cumulative incidences of NRM after 100 days, 1 year, and 5 years were 15.8%, 26.3%, and 33.9% (Figure 1A, Table 2). In total, 44 patients died of nonrelapse causes during the entire observation time. Among those were 26 infections, 5 cases of acute and 4 causes of chronic GVHD, 3 cases of fatal organ toxicity, 1 graft failure, 1 hemorrhage, 1 myocardial infarction, 2 secondary neoplasias, and 1 unknown cause (Table 3). None of the examined factors for univariate and multivariate analyses proved statistically significant for the incidence of NRM.
Causes of death
| . | All patients, n = 133 . | AML/MDS patients, n = 81 . |
|---|---|---|
| Total | 69 | 44 |
| Relapse | 25 | 17 |
| NRM | 44 | 27 |
| Infection | 26 | 19 |
| aGVHD | 5 | 5 |
| cGVHD | 4 | |
| Hemorrhage | 1 | |
| Graft failure | 1 | |
| Organ toxicity | 3 | 1 |
| Secondary neoplasia | *2 | 1 |
| Myocardial infarction | 1 | |
| Unknown | 1 | 1 |
| . | All patients, n = 133 . | AML/MDS patients, n = 81 . |
|---|---|---|
| Total | 69 | 44 |
| Relapse | 25 | 17 |
| NRM | 44 | 27 |
| Infection | 26 | 19 |
| aGVHD | 5 | 5 |
| cGVHD | 4 | |
| Hemorrhage | 1 | |
| Graft failure | 1 | |
| Organ toxicity | 3 | 1 |
| Secondary neoplasia | *2 | 1 |
| Myocardial infarction | 1 | |
| Unknown | 1 | 1 |
Secondary neoplasias: bronchial, pancreatic.
The FBM protocol was fairly well tolerated. Twenty-four cases of severe toxicity (Score 3 and 4 according to Bearman et al13 ) have been observed (Table 4). Despite a high degree of renal complications, none of the patients experienced permanent dialysis treatment due to terminal renal insufficiency. The cases of fatal organ toxicity included one patient with ALL and severe leukencephalopathy most likely after initial multiple intrathecal application of chemotherapeutics and putative additional central nervous system (CNS) toxicity due to fludarabine and CsA. The second patient experienced liver failure either by venoocclusive disease or septic complications with suspected preexisting liver insufficiency. The third patient suffered from a widespread relapse of a T-NHL prior to allogeneic HCT. After reversible cardiovascular complications during extensive pretreatment, the patient died by toxicity-related multiorgan failure complicated by sepsis despite successful engraftment.
Although not included in the study, 13 patients with prior autologous or allogeneic HCT were also treated with the FBM protocol. All of them presented with advanced disease at the time of allogeneic HCT. The toxicity of the conditioning for this particular group was comparable with the study group, resulting in only one case with renal and mucosal grade 3 toxicity. One patient in progressive sAML after first allogeneic HCT with fludarabine died by overwhelming sepsis before reconstitution of granulopoiesis. Nine of these high-risk patients with advanced disease died during follow-up (5 infections, 4 relapses, 1 acute GVHD).
Cumulative incidences for acute GVHD grade III and IV were evaluated as competing risk for death by any cause. In total 31 cases (rate: 23.3%) were reported including 12 cases (9.0%) of grade 4 acute GVHD (Figure 1C, Table 5). Interestingly, the incidence rates of severe acute GVHD III/IV were only 16.9% in unrelated and 29.4% in related donor HCT (Table 5), probably because of the use of ATG. Further analysis of known risk factors for the incidence of acute grade II to IV GVHD revealed only a trend (P = .07) for reduced problems using unrelated donors and ATG, respectively.
Acute GVHD outcome of patient cohort
| GVHD stage . | No. of patients, % (range) . |
|---|---|
| II-IV | 44.4 (36.7-53.7) |
| Related | 51.5 (40.9-64.8) |
| Unrelated | 36.9 (26.9-50.7) |
| III-IV | 23.3 (17.1-31.7) |
| Related | 29.4 (20.4-42.5) |
| Unrelated | 16.9 (9.9-29.0) |
| IV | 9.0 (5.3-15.5) |
| Related | 8.8 (4.1-18.9) |
| Unrelated | 9.2 (4.3-19.8) |
| GVHD stage . | No. of patients, % (range) . |
|---|---|
| II-IV | 44.4 (36.7-53.7) |
| Related | 51.5 (40.9-64.8) |
| Unrelated | 36.9 (26.9-50.7) |
| III-IV | 23.3 (17.1-31.7) |
| Related | 29.4 (20.4-42.5) |
| Unrelated | 16.9 (9.9-29.0) |
| IV | 9.0 (5.3-15.5) |
| Related | 8.8 (4.1-18.9) |
| Unrelated | 9.2 (4.3-19.8) |
Extensive chronic GVHD was evaluated in the same way showing a cumulative incidence of 32.3% after 1 year and 33.1% after 2 years (Figure 1D). Again, the incidence was less in the unrelated setting (13.8%) than in related donor HCT (51.5%; Table 6). The significantly reduced risk of extensive GVHD in allografting from an unrelated donor could also be demonstrated in multivariate analysis (P = .001). Interestingly, female donor sex was associated with a significantly higher risk of chronic GVHD (P = .002) in multivariate analysis. No differences according to the 2 prophylactic regimens (CsA/mMTX and CsA/MMF) could be demonstrated for the incidence of acute (P = .58) and chronic extensive GVHD (P = .533) in univariate analysis. Although direct comparison of both regimens is difficult because of their subsequent use and the addition of ATG according to the donor, the lack of a detectable difference argues also for a strong impact of ATG for the incidence of GVHD, irrespective of the used prophylaxis.
Chronic GVHD outcome of patient cohort
| . | 1 year . | 2 years . | P . |
|---|---|---|---|
| Chronic GVHD (extensive) | 32.3 (25.3-41.3) | 33.1 (26.0-42.1) | |
| Donor | |||
| Related | 51.5 (40.9-64.8) | 51.5 (40.9-64.8) | |
| Unrelated | 12.3 (6.4-23.6) | 13.8 (7.6-25.4) | .001** |
| GVHD prophylaxis | |||
| CsA/MMF | 30.0 (22.6-39.9) | 30.9 (23.4-40.9) | |
| CsA/mMTX | 43.5 (27.3-69.3) | 43.5 (27.3-69.3) | .53* |
| . | 1 year . | 2 years . | P . |
|---|---|---|---|
| Chronic GVHD (extensive) | 32.3 (25.3-41.3) | 33.1 (26.0-42.1) | |
| Donor | |||
| Related | 51.5 (40.9-64.8) | 51.5 (40.9-64.8) | |
| Unrelated | 12.3 (6.4-23.6) | 13.8 (7.6-25.4) | .001** |
| GVHD prophylaxis | |||
| CsA/MMF | 30.0 (22.6-39.9) | 30.9 (23.4-40.9) | |
| CsA/mMTX | 43.5 (27.3-69.3) | 43.5 (27.3-69.3) | .53* |
Data are percentages (ranges) of patients.
CI indicates 95% confidence interval.
Univariate analysis (Cox proportional hazards regression models).
Multivariate analysis (Cox proportional hazards regression models).
Extensive chronic GVHD can result in reduced quality of life especially in long-term survivors after allogeneic HCT. Therefore 42 patients with extensive cGVHD and sufficient follow-up data could be analyzed for their performance status. Fourteen patients died during follow-up, 5 of them with cGVHD contributing to the dismal outcome. Twenty-eight patients are alive, among these are 4 with complete and 2 with partial resolution of their cGVHD. Despite persistence of signs of cGVHD, 20 patients were reported with Karnofsky index 90% or more.
Overall and event-free survival
The entire study cohort showed a 1-, 3-, and 5-year overall survival (OS) of 61.7%, 53.0%, and 46.1%. Event-free survival (EFS) at the same time points was 56.4%, 46.4%, and 41.9% (Table 2). In particular, all CML patients who received transplants in CP1 (n = 8) remained free of disease for the entire observation period. Only 1 patient died of bronchial carcinoma more than 3.5 years after allogeneic HCT.
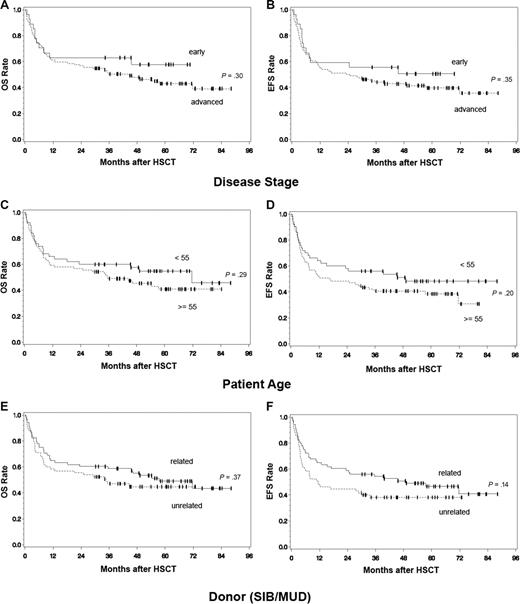
For the entire patient cohort, there was no statistically significant difference between disease stages regarding overall and event- free survival (Table 2). Patients with early disease experienced a 1-, 3-, and 5-year OS of 63.0%, 63.0%, 57.7% or an EFS of 59.3%, 55.6%, and 50.5%. For patients with advanced stages, OS was 61.3%, 50.4%, 43.0% with corresponding EFS of 55.7%, 44.0%, 39.6% (Figure 2A,B). Similarly, patients 55 years of age or older showed no significant difference in OS and EFS (59.0%, 48.9%, 40.8%) than younger ones (66.0%, 60.0%, 54.8%; Figure 2C,D). There was also no difference between patients receiving transplants from a related or unrelated donor (Figure 2E,F).
Overall and event-free survival of entire patient cohort. Total patient cohort (n = 133): Overall survival (OS) (A) and event-free survival (EFS) (B) according to disease status at time of HCT (advanced vs early), OS (C) and EFS (D) according to patient age (< 55 vs ≥ 55 years), OS (E) and EFS (F) according to donor choice (related vs unrelated), P values: univariate analysis (Cox proportional hazards regression model).
Overall and event-free survival of entire patient cohort. Total patient cohort (n = 133): Overall survival (OS) (A) and event-free survival (EFS) (B) according to disease status at time of HCT (advanced vs early), OS (C) and EFS (D) according to patient age (< 55 vs ≥ 55 years), OS (E) and EFS (F) according to donor choice (related vs unrelated), P values: univariate analysis (Cox proportional hazards regression model).
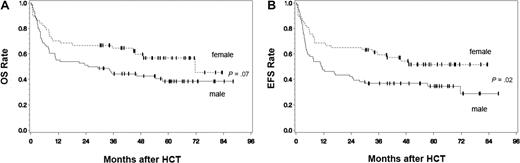
Interestingly, allografting from female donors resulted in significantly increased event-free survival after 5 years (51.7%, CI: 37.9-65.5%) in contrast to male donors (34.6%, CI: 23.6-45.6%, P = .02) (Figure 3B). This difference could also be found in multivariate analysis (P = .04) and correlates with the already mentioned higher cumulative incidence rate of extensive GVHD in patients with female (52.6%, CI: 41.1-67.3%) than with male donors (18.4%, CI: 11.5-29.6%, P = .002). Increased EFS rates in patients with female donors was followed by a trend for an improved OS (56.8% vs 38.4% for male donors after 5 years, P = .07; Figure 3A).
Overall and event-free survival of entire patient cohort according to the donor sex. Total patient cohort (n = 133): OS (A) and EFS (B) of patients according to the sex of the stem cell donor (female vs male), P values: univariate analysis (Cox proportional hazards regression model).
Overall and event-free survival of entire patient cohort according to the donor sex. Total patient cohort (n = 133): OS (A) and EFS (B) of patients according to the sex of the stem cell donor (female vs male), P values: univariate analysis (Cox proportional hazards regression model).
Considering the median long-term follow-up of the survivors of 58 months, data regarding their quality of life are of particular interest. A Karnofsky index of 90% or more was reported by 45 patients from a total of 53 patients with sufficient data about their performance status. The remaining 8 patients with reduced performance status are all experiencing extensive chronic GvHD.
Because the vast majority of included patients (n = 81) presented with AML/MDS, a subgroup analysis of this group was performed. These patients experienced 1-, 3-, and 5-year OS and EFS of 54.3%, 46.5%, 44.8% and 46.9%, 40.4%, 40.4%, respectively (Table 7). In particular, advanced cases showed an OS of 54.7%, 44.6%, 42.4% while patients with early disease experienced an OS of 52.9% at 1-, 3-, and 5-year intervals (Figure 4A). Corresponding EFS for advanced disease was 46.9%, 38.6%, 38.6% for the 3 time points and 47.1% for the entire period in early cases (Figure 4B). These results are particularly encouraging, because the AML/MDS group with advanced malignancies consisted mainly of patients with active disease at the time of allogeneic HCT. In univariate analyses, including patients' age and donor choice, no significant factors regarding OS and EFS of the AML/MDS patients could be found. There was a trend for a reduced EFS in patients receiving their graft from a female donor (P = .07).
Outcome of AML/MDS patients (n = 81)
| . | Day 100 . | 1 year . | 3 years . | 5 years . | P . |
|---|---|---|---|---|---|
| Overall survival | 81.5 (73.0-89.9) | 54.3 (43.5-65.2) | 46.5 (35.5-57.4) | 44.8 (33.7-55.8) | |
| Disease stage at HSCT | |||||
| Early | 82.4 (64.2-100.0) | 52.9 (29.2-76.7) | 52.9 (29.2-76.7) | 52.9 (29.2-76.7) | |
| Advanced | 81.3 (71.7-90.8) | 54.7 (42.5-66.9) | 44.6 (32.3-57.0) | 42.4 (29.9-54.9) | .69* |
| Event-free survival | 77.8 (68.7-86.8) | 46.9 (36.0-57.8) | 40.4 (29.6-51.2) | 40.4 (29.6-51.2) | |
| Disease stage at HSCT | |||||
| Early | 82.4 (64.2-100.0) | 47.1 (23.3-70.8) | 47.1 (23.3-70.8) | 47.1 (23.3-70.8) | |
| Advanced | 76.6 (66.2-86.9) | 46.9 (34.6-59.1) | 38.6 (26.5-50.7) | 38.6 (26.5-50.7) | .66* |
| Relapse | 1.2 (0.2-8.7) | 14.8 (8.8-25.0) | 19.9 (12.8-30.9) | 21.7 (14.2-33.1) | |
| NRM | 17.3 (10.7-27.8) | 30.9 (22.3-42.8) | 33.6 (24.7-45.7) | 33.6 (24.7-45.7) |
| . | Day 100 . | 1 year . | 3 years . | 5 years . | P . |
|---|---|---|---|---|---|
| Overall survival | 81.5 (73.0-89.9) | 54.3 (43.5-65.2) | 46.5 (35.5-57.4) | 44.8 (33.7-55.8) | |
| Disease stage at HSCT | |||||
| Early | 82.4 (64.2-100.0) | 52.9 (29.2-76.7) | 52.9 (29.2-76.7) | 52.9 (29.2-76.7) | |
| Advanced | 81.3 (71.7-90.8) | 54.7 (42.5-66.9) | 44.6 (32.3-57.0) | 42.4 (29.9-54.9) | .69* |
| Event-free survival | 77.8 (68.7-86.8) | 46.9 (36.0-57.8) | 40.4 (29.6-51.2) | 40.4 (29.6-51.2) | |
| Disease stage at HSCT | |||||
| Early | 82.4 (64.2-100.0) | 47.1 (23.3-70.8) | 47.1 (23.3-70.8) | 47.1 (23.3-70.8) | |
| Advanced | 76.6 (66.2-86.9) | 46.9 (34.6-59.1) | 38.6 (26.5-50.7) | 38.6 (26.5-50.7) | .66* |
| Relapse | 1.2 (0.2-8.7) | 14.8 (8.8-25.0) | 19.9 (12.8-30.9) | 21.7 (14.2-33.1) | |
| NRM | 17.3 (10.7-27.8) | 30.9 (22.3-42.8) | 33.6 (24.7-45.7) | 33.6 (24.7-45.7) |
Data are percentages (ranges).
CI indicates 95% confidence interval.
Univariate analysis (Cox proportional hazards regression models).
Overall survival, event-free survival, and relapse rate of AML and MDS patients. AML/MDS patients (n = 81): OS (A) and EFS (B) according to disease status at time of HCT (advanced vs early), P values: univariate analysis (Cox proportional hazards regression model), (C) cumulative incidence of relapse according to disease status at time of HCT (advanced vs early).
Overall survival, event-free survival, and relapse rate of AML and MDS patients. AML/MDS patients (n = 81): OS (A) and EFS (B) according to disease status at time of HCT (advanced vs early), P values: univariate analysis (Cox proportional hazards regression model), (C) cumulative incidence of relapse according to disease status at time of HCT (advanced vs early).
In total, 17 patients relapsed after HCT. The observed cumulative relapse rates after 1, 3, and 5 years were 14.8%, 19.9%, and 21.7% (Table 2). Interestingly, there were no posttransplantation relapses in patients who presented with early disease at the time of allogeneic HCT (Figure 4C). The cumulative incidence of NRM (n = 27) after 100 days and 1 and 5 years were 17.3%, 30.9%, and 33.6% (Table 2) with infections being the leading causes of death (Table 3). No prognostic factors for the incidence of relapse or nonrelapse mortality could be found in univariate analysis for the AML/MDS patients treated with FBM conditioning.
To further determine the efficacy of FBM conditioning for active myeloid disease we analyzed the subgroup of AML/MDS patients with circulating blasts at the time of allogeneic HCT. In total, 33 patients could be identified with blasts in the peripheral blood (median: 21.1%, range 1%-98%) and median marrow blast counts of 51,9% (range: 8%-98%). The OS rates in this group after 1, 3, and 5 years were 54.5%, 48.5%, and 44.4% (Figure 5A), the EFS rates were 42.4%, 36.4%, and 36.4%, respectively (Figure 5B).
Overall and event free survival of AML and MDS patients with blasts in the peripheral blood at time of HCT. AML/MDS patients: OS (A) and EFS (B) of the subgroup of patients with blasts in the peripheral blood at the time of HCT (n = 33).
Overall and event free survival of AML and MDS patients with blasts in the peripheral blood at time of HCT. AML/MDS patients: OS (A) and EFS (B) of the subgroup of patients with blasts in the peripheral blood at the time of HCT (n = 33).
Discussion
In this report we extend our previous data of patients undergoing allogeneic HCT after FBM conditioning.6,12 The FBM protocol includes fludarabine, BCNU, and melphalan as a combination of lymphotoxic and myelotoxic agents. Therefore, the high degree of complete chimerism after engraftment together with the very low incidence of graft failure underscores the substantial regimen-related immuno- and myelosuppressive characteristics, making it applicable for safe allografting from both related and unrelated donors. In addition, FBM conditioning results in high CR rates with acceptable toxicity in older patients or patients with comorbidities. The high antileukemic activity of FBM could be proved not only in patients with early disease stages, but showed also encouraging long-term effectiveness even in cases of advanced or active disease.
In recent years, several groups including ours have reported on the feasibility of reduced-intensity conditioning (RIC) for allografting elderly patients or patients in poor medical condition and with considerable comorbidities.6,7,16-20 Conditioning intensity among separate protocols could be defined as minimal, reduced, and conventional high dose.1,2 In this regard the regimen-induced dose-dependent immuno- and myelosuppression correlates with its antileukemic activity, but increasing dose intensity confers also higher risks for severe organ toxicity and nonrelapse mortality.
How can the intensity of FBM be placed in this dose spectrum of conditioning regimens? Characteristically, the FBM preparative regimen shows effective donor immunosuppression, resulting in a very low incidence of primary graft failures. In addition, the dose intensity results in a high degree of complete chimerism. Disease control by FBM is similar to conventional high-dose conditioning approaches while regimen-related toxicity and NRM remain comparable with regimens with dose-reduced conditioning. Therefore FBM can be regarded as a toxicity-reduced regimen with strong myelosuppressive capacity, placing this protocol between conventional high-dose/myeloablative and dose-reduced intensity (eg, fludarabine/melphalan or fludarabine/busulfan) approaches. Because FBM shows dose intensity close to conventional high-dose protocols, the term reduced-intensity conditioning (RIC) is not applicable for this type of preparative regimen. Nevertheless, we think FBM is well suited for the treatment of older patients, who cannot tolerate substantial toxicity problems. Therefore we consider FBM as toxicity-reduced conditioning, to emphasize the clinical applicability of this protocol for patients not eligible for conventional high-dose conditioning.
In AML/MDS patients, reduced-intensity conditioning with fludarabine and melphalan (140-180 mg/m2) is known to result in 3-year OS and EFS of 35% to 41% and 32% to 37%, respectively, while NRM has been reported with 19% after 1 and 35% after 3 years.21,22 Representative clinical trials using fludarabine and busulfan in the same patients with myeloid malignancies report 2-year OS of 47% to 49%, EFS of 43% to 49% and NRM of 8%.23
Additional studies with AML and/or MDS patients in which known myeloablative regimens have been modified for reduction in toxicity by using targeted busulfan and cyclophosphamide have been published.23,24 Although AML/MDS patients show comparable 2-year OS (50%) and EFS (45%) as in fludarabine/busulfan RIC regimes, NRM (22%) was substantially higher.23 Patients with MDS experience a 3-year EFS of 56% to 59% and NRM of 28% to 30%.24 These data show disease control with FBM conditioning at least as effective as in published RIC protocols, while NRM remains lower than in conventional high-dose/myeloablative conditioning.
Toxicity-reduced conditioning protocols were initially designed for successful engraftment, with considerable antileukemic activity suited for allogeneic HCT for comorbid or older patients. Additional treatment options for these patients are mandatory, because especially in AML patients older than 60 years, conventional induction and consolidation therapy results in 3-year OS of 18% for standard-risk and only 5% for high-risk cytogenetics.25 In accordance with other trials using toxicity-reduced conditioning,26 allogeneic HCT with FBM results in improved long-term survival for patients older than 55 years, approaching similar outcomes observed for younger patients. Therefore, allogeneic HCT with FBM can be firmly expected to improve overall survival in this particular critical patient group.
Another important clinical problem addressed in several trials with reduced-toxicity conditioning is the antileukemic activity of the protocols against advanced or active disease. In fact, a variety of nonmyeloablative protocols showed effective disease control only in early disease. AML patients in CR1 treated with fludarabine/total body irradiation (TBI) showed an impressively low day 100 NRM of 3%, with a 2-year OS of 51%. But patients with advanced disease had a 2-year OS of only 28%, with better survival in patients with unrelated compared with related donors.9 A longer follow-up of the AML/MDS patients at the M. D. Anderson Cancer Center (Houston, TX) using fludarabine/melphalan regimens resulted in 5-year OS of approximately 42% in patients with complete remission and of only approximately 24% in patients with active disease at the time of transplantation.27 In a similar protocol, van Besien et al28 added alemtuzumab (5 × 20 mg) to the conditioning regimen for the treatment of standard and high-risk AML/MDS patients. In this cohort 1-year OS and EFS of patients with advanced disease was only 37% and 25%. This reduced 1-year survival was due to a high NRM (39%) and relapse rate. Similar results with fludarabine/melphalan/alemtuzumab conditioning were obtained by Tauro et al21,22 : For patients with relapsed/refractory AML a 3-year EFS of only 20% was reported.
Shimoni et al23 showed that increased dose intensity among different RIC protocols is extremely critical for control of advanced disease. In this study using fludarabine/busulfan regimens, the doubling of the busulfan dose from 6.4 mg/kg to 12.8 mg/kg resulted in improved 2-year OS from 0% to 39%. A recent trial particularly addressing allogeneic HCT in refractory acute myeloid leukemia used an induction therapy with fludarabine, cytarabine, and amsacrine followed by a cyclophosphamide/TBI RIC-protocol.10 After 4 years, OS was 32% and EFS was 30%. As expected, the relapse-related death rate at 1 year was 28.7% in this high-risk group while NRM was only 17.2%.
Among the AML/MDS patients with advanced disease in our study, the particular subgroup of patients with active myeloid malignancies (circulating blasts in the peripheral blood and median blast marrow count of 52%) at the time of allogeneic HCT deserve special attention. Prior data regarding allogeneic HCT with reduced-intensity conditioning in patients with active disease emphasize the dismal prognosis.4,7 Allogeneic HCT with FBM conditioning results in a 5-year overall survival of more than 40%. These data underscore the high antileukemic efficacy of FBM and are comparable with combined approaches using induction therapy before allogeneic HCT with RIC.10
These trials prove that the dose intensity of toxicity-reduced conditioning is critical for disease control. However, long-term survival with reduced NRM after allogeneic HCT even in older patients can be achieved by “fine-tuning” dosages and drugs for the conditioning regimen. In addition to dose intensity of cytotoxic substances, monitoring the alloreactivity provided by the stem cell graft, GVHD prophylaxis, and preemptive donor lymphocyte infusions according to the treated malignancy should be considered.
Two interesting results from this trial might have an impact in further clinical practice of allogeneic HCT. First, the use of unrelated donors together with ATG-F as additional GVHDprophylaxis resulted in dramatically decreased incidences of chronic GVHD, with a nonsignificant slight increase in EFS and relapse mortality and unaffected NRM and OS. Because the particular impact of ATG-F or the donor choice for this outcome cannot be further statistically analyzed, a general statement for the role of ATG in allogeneic HCT is not possible. Anyway, the use of ATG-F in unrelated donors contributes to the safety of nonrelated HCT, making even the choice of a particular GVHD prophylaxis regimen less important. Because it results in comparable OS rates to related HCT, this therapeutic combination might help to offer allogeneic HCT as putative curative treatment to patients without related donors. Second, patients receiving their graft from female donors experienced an improved EFS, most likely due to more cGVHD, which also resulted in superior OS. These data could help to define the best donor in situations where high alloreactivity of the graft is wanted, for example, in uncontrolled disease.
Of particular note is the high rate of NRM and relapse in our 13 patients treated after prior autologous or allogeneic HCT. In an attempt to further reduce the toxicity for these heavily pretreated patients, our current protocol for similar cases includes allogeneic HCT after conditioning with fludarabine and thiotepa.
By adding FBM to the dose-intensity spectrum of conditioning regimens, we emphasize an algorithm in which preparative regimens used for allogeneic HCT should be applied according not only to age and comorbidities of the patient, but also to the characteristics and risk profile of the underlying malignancy. In this regard, extended immunodeficiency and prior infectious complications in malignant myeloid diseases need particular consideration, while the choice of donor (related vs unrelated) appears to be much less important for successful allografting.
In conclusion, the FBM protocol can be viewed as a toxicity-reduced preparative regimen combining effective disease control particularly for advanced disease stages with low nonrelapse mortality, making this protocol feasible even for older patients or patients with a higher degree of comorbidities.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful for the dedicated work of the transplantation team from transplant ward Löhr and to E. Lenartz for transplant coordination, E. Samek for excellent technical assistance, I. Matt for documentation work, and Prof R. Mertelsmann for continuous support.
Authorship
Contribution: R.M. analyzed data and wrote the paper; K.P. treated patients, analyzed data, and wrote the paper; J.H. and H.B. treated patients and analyzed data; G.I. analyzed data; A.S. treated patients; E.H. designed the study and treated patients; J.F. designed the study, treated patients, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. Finke, MD, Albert-Ludwigs University Medical Center, Department of Haematology and Oncology, Hugstetter Strausse 55, D-79106 Freiburg, Germany; e-mail: juergen.finke@uniklinik-freiburg.de.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal