In this issue of Blood, Qiang and colleagues show that increasing Wnt signaling within the myeloma bone microenvironment inhibits myeloma bone disease and consequently reduces tumor burden.
For many years, the osteoclast was thought to be the main culprit in the development of the destructive osteolytic bone disease that is a characteristic feature of multiple myeloma. Only more recently were suppression of osteoblastogenesis and bone formation found to contribute to the systemic bone loss and osteolytic bone lesions associated with myeloma bone disease. Current therapies targeting the osteoclast are effective at preventing further bone destruction, but cannot replace bone that has already been lost. Therefore, targeting bone formation in multiple myeloma is an attractive therapeutic approach.
Compelling evidence for the critical role Wnt signaling plays in promoting osteoblast differentiation and bone formation, and the discovery of Dickkopf1 as a mediator of the reduction in osteoblastic bone formation in multiple myeloma, both identify the Wnt signaling pathway as a potential therapeutic target in multiple myeloma.1-3 Qiang and colleagues took 2 complementary approaches to exploring this new avenue: overexpression of Wnt3A in myeloma cells, and systemic Wnt3A treatment. Both approaches use the severe combined immunodeficient (SCID) hu myeloma model, in which human myeloma cell growth is restricted to human bone implanted into immunodeficient mice. Under normal circumstances, the SCID-hu myeloma model is associated with tumor growth and osteolysis of the human bone fragment. However, increasing Wnt signaling with Wnt3A resulted in a reduction in tumor burden, an increase in bone mineral density, and a decrease in the osteoclast:osteoblast ratio. These data support an earlier study by Edwards et al in which systemic activation of Wnt signaling with lithium chloride was shown to prevent myeloma bone disease and indirectly reduce tumor burden in bone in the 5TGM1 murine model of myeloma.4
The direct effect of Wnt signaling on myeloma cells remains controversial. Qiang and colleagues demonstrate that although Wnt3A activates Wnt signaling in myeloma cells, this is not associated with an increase in proliferation, either in vitro or in vivo. This eliminates the possibility of direct proliferative effects through Wnt signaling in myeloma cells in this model. Although bone formation is not directly assessed, evidence is provided to support a direct effect of Wnt3A in increasing Wnt signaling in cells of the osteoblast lineage. Taken together, the results presented by Qiang et al suggest that, within the bone microenvironment, Wnt3A acts to promote osteoblast differentiation and suppress osteoclastogenesis. This results in inhibition of myeloma bone disease and consequent indirect suppression of tumor growth. These studies advance our knowledge of myeloma bone disease, and raise questions regarding the balance of agonists and antagonists of the Wnt signaling pathway in myeloma, and the function of Wnt signaling in myeloma cells.
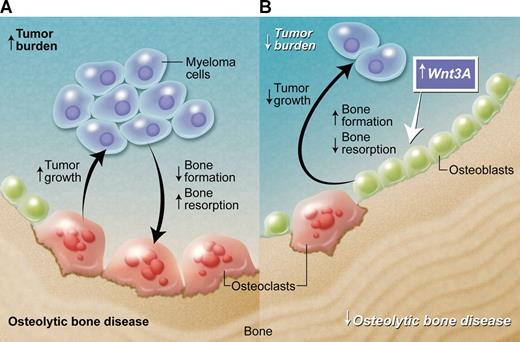
Effect of Wnt3A in the bone microenvironment in multiple myeloma. (A) Myeloma cells promote the development of the associated bone disease, and the bone disease promotes tumor growth and survival, resulting in a vicious cycle of increased tumor burden and increased osteolytic bone disease. (B) Wnt3A enhances Wnt signaling in the bone marrow microenvironment. This has no direct effect on myeloma-cell growth, but acts to increase bone formation and decrease bone resorption. This inhibits myeloma bone disease and, consequently, indirectly reduces tumor burden in bone. Professional illustration by Alice Chen.
Effect of Wnt3A in the bone microenvironment in multiple myeloma. (A) Myeloma cells promote the development of the associated bone disease, and the bone disease promotes tumor growth and survival, resulting in a vicious cycle of increased tumor burden and increased osteolytic bone disease. (B) Wnt3A enhances Wnt signaling in the bone marrow microenvironment. This has no direct effect on myeloma-cell growth, but acts to increase bone formation and decrease bone resorption. This inhibits myeloma bone disease and, consequently, indirectly reduces tumor burden in bone. Professional illustration by Alice Chen.
The conclusions by Qiang and colleagues provide strong support for the potential of targeting the Wnt signaling pathway within the bone marrow microenvironment for the treatment of myeloma bone disease. Furthermore, they highlight the complex interdependence between myeloma cells and host cells of the bone marrow niche, along with the importance of studying myeloma within the appropriate microenvironment.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal