Abstract
In lymph node (LN) proliferation centers in chronic lymphocytic leukemia (CLL), the environment protects from apoptotic and cytotoxic triggers. Here, we aimed to define the molecular basis for the increased drug resistance and searched for novel strategies to circumvent it. The situation in CLL LN could be mimicked by prolonged in vitro CD40 stimulation, which resulted in up-regulation of antiapoptotic Bcl-xL, A1/Bfl-1, and Mcl-1 proteins, and afforded resistance to various classes of drugs (fludarabine, bortezomib, roscovitine). CD40 stimulation also caused ERK-dependent reduction of Bim-EL protein, but ERK inhibition did not prevent drug resistance. Drugs combined with sublethal doses of the BH3-mimetic ABT-737 displayed partial and variable effects per individual CD40-stimulated CLL. The antiapoptotic profile of CD40-triggered CLL resembled BCR-Abl–dependent changes seen in chronic myeloid leukemia (CML), which prompted application of c-Abl inhibitors imatinib or dasatinib. Both compounds, but especially dasatinib, prevented the entire antiapoptotic CD40 program in CLL cells, and restored drug sensitivity. These effects also occurred in CLL samples with dysfunctional p53. Importantly, ex vivo CLL LN samples also displayed strong ERK activation together with high Bcl-xL and Mcl-1 but low Bim levels. These data indicate that CLL cells in chemoresistant niches may be sensitive to therapeutic strategies that include c-Abl inhibitors.
Introduction
Chronic lymphocytic leukemia (CLL) is a CD5+ B-cell malignancy that is still considered incurable, although novel treatment combinations of monoclonal antibodies and chemotherapy1 seem promising. Many patients eventually develop drug resistance through several pathways, including mutation of the p53 tumor suppressor gene, or involving the gene encoding the ataxia telangectasia mutated (ATM), which is a kinase required for p53 function. Such genetic lesions are uncommon in CLL at diagnosis, but increase in frequency as the disease progresses.2 Since the cytoreductive activity of most current chemotherapeutic agents requires functional p53, loss of p53 is associated with drug resistance and poor prognosis.3 Because of these aspects, different agents with p53-independent modes of action are clearly needed.
CLL has been considered a smoldering disease characterized by long-lived tumor cells arrested in the G0/G1 phase of the cell cycle and possessing intrinsic apoptosis defects.4 This concept was largely based on analyses of peripheral blood–derived CLL cells. A study of in vivo cellular kinetics, however, suggested that CLL is a dynamic disease with substantial proliferation rates as well as increased death rates compared with normal B cells.5 Prior to this, it was already known that proliferation and especially increased survival of the malignant B cells may not result primarily from intrinsic defects, but appear to depend largely on interactions with micro-environmental bystander cells. Interactions between CLL cells and follicular dendritic cells, bone marrow stromal cells, IL-6–producing endothelial cells, stromal cell–derived factor (SDF)–producing nurselike cells, or CD40L expressing CD4+ T cells6-9 have been shown to increase the apoptotic threshold in vitro. In a recent comparative survey of apoptosis regulatory genes and proteins in neoplastic B cells derived from CLL lymph node (LN) proliferation centers and from peripheral blood,10 we observed specific changes including increased expression of antiapoptotic proteins such as Mcl-1, Bcl-XL, and A1/Bfl-1 in LN cells. Extended cell survival of tumor cells within the LN microenvironment may create an intracellular milieu permissive for genetic instability and for the accumulation of gene mutations that favors disease progression. Furthermore, these micro-environmental interactions may provide a safe haven from cytotoxic anticancer drugs, thus serving as a tumor reservoir from which relapse occurs (reviewed by Pedersen and Reed11 ). This concept is supported by the observation that prolonged CD40 activation, which to a large extent recapitulates the antiapoptotic expression profile of LN-derived CLL cells, renders CLL cells resistant to current chemotherapeutics.12,13 The currently widely applied drug fludarabine relies on an intact p53 response, which induces expression of the Bcl-2 member Puma, thereby triggering apoptosis.14-16 Alternative, p53-independent drugs such as the proteasome inhibitor bortezomib or the cyclin-dependent kinase inhibitor roscovitine engage other proapoptotic Bcl-2 members such as Noxa and Bim. Especially Bim is a potent pro-apoptosis member of the BH3-only subgroup of the Bcl-2 family, engaged by a variety of apoptotic triggers.17-20 A potential means of suppressing the lethal capacity of Bim involves the prosurvival kinase ERK. In model systems, activation of ERK leads to phosphorylation and subsequent proteasomal degradation of the Bim-EL splice variant.21,22
In the present study we used in vitro CD40 stimulation as a model for chemoresistant LN CLL, and searched for means to circumvent it. CD40 stimulation of CLL cells strongly induced Bcl-XL, Mcl-1, and A1/Bfl-1 proteins, resulting in a broad drug resistance. Various aspects of this antiapoptotic program also occur in chronic myeloid leukemia (CML), a disease for which current treatment includes kinase inhibitors that were developed to target BCR-Abl signaling.23 Therefore, we next applied the c-Abl inhibitors imatinib (Gleevec, STI-571) or dasatinib (Sprycel, BMS-354825) in conjunction with CD40. Both drugs caused a profound reversal of the protective CD40 effects, and restored drug sensitivity. Probing of LN CLL samples demonstrated that in these protective niches similar prosurvival signaling pathways are active as upon CD40 triggering in vitro. Collectively, these data suggest that CLL cells residing in LN might be therapeutically targeted by drug combinations that include c-Abl inhibitors.
Methods
Patient material
Patient material (Table 1) was obtained after routine diagnostic or follow-up procedures at the departments of Hematology and Pathology of the Academic Medical Center (AMC) Amsterdam. This study was conducted and approved by the AMC Medical Committee on Human Experimentation. Informed consent was obtained in accordance with the Declaration of Helsinki. LN material, diffusely infiltrated by CLL cells, was freshly frozen in liquid nitrogen directly after surgical removal. Immunohistochemical analysis of these lymph nodes revealed that greater than 90% of the tissue consisted of tumor cells.10 Peripheral blood (PB) mononuclear cells (PBMCs) of patients with CLL, obtained after Ficoll density gradient centrifugation (Pharmacia Biotech, Roosendaal, The Netherlands) were frozen in Iscove modified Dulbecco medium (IMDM) supplemented with l-glutamine, 25 mM HEPES (Lonza Verviers SPRL, Verviers, Belgium), containing 2 mM l-glutamine (Invitrogen, Carlsbad, CA), 50 mg gentamycin (Invitrogen), 3.57 × 10−4% (vol/vol) β-mercaptoethanol (Merck, Darmstadt, Germany), 10% dimethyl sulphoxide (DMSO; Sigma-Aldrich, St Louis, MO), and 15% fetal calf serum (FCS; ICN Biomedicals, Meckenheim, Germany), and stored in liquid nitrogen. Expression of CD5 and CD19 (antibodies obtained from Beckton Dickinson [BD] Biosciences, San Jose, CA) on leukemic cells was assessed by flow cytometry (FACSCalibur; BD Biosciences) and analyzed with CellQuest software (BD Biosciences).
Patient characteristics (peripheral blood samples)
| Patient . | Rai stage . | WBC, 109/L . | Lymphocytes, % . | CD5+/ CD19+, %* . | CD3+, % . | IgVH† mutational status . | p53 status‡ . | CD38 (CD20+), % . | Chromosomal abnormalities§ . |
|---|---|---|---|---|---|---|---|---|---|
| CLL 01 | II | 113.0 | 90.0 | 88.3 | 4.4 | + | Functional | 81 | None |
| CLL 07 | I | 93.3 | 86.9 | 96.8 | 2.4 | + | Functional | 87 | 11q− |
| CLL 08 | 0 | 58.5 | 91.0 | 92.7 | 4.3 | + | ND | 24 | None |
| CLL 11 | II | 49.3 | 88.8 | 91.4 | 5.5 | − | Functional | 92 | 13q− |
| CLL 12 | I | 72.2 | 86.3 | 93.0 | 5.0 | + | Functional | 33 | None |
| CLL 16 | II | 80.1 | 73.7 | 92.0 | 6.0 | + | ND | 39 | 13q− |
| CLL 17 | II | 102.0 | 92.1 | 93.3 | 4.1 | − | ND | 47 | None |
| CLL 18 | 0 | 48.0 | 89.2 | 90.5 | 7.7 | + | ND | 29 | ND |
| CLL 20 | II | 61.4 | 89.0 | 95.0 | 4.0 | + | Functional | 20 | ND |
| CLL 21 | IV | 60.6 | 89.8 | 86.7 | 3.1 | − | ND | 24 | ND |
| CLL 22 | I | 60.1 | 94.4 | 81.7 | 6.5 | − | Dysfunctional | 44 | 17p− |
| CLL 23 | IV | 137.0 | 94.8 | 83.5 | 3.8 | + | Dysfunctional | 57 | 17p−;13q− |
| CLL 25 | I | 79.0 | 93.3 | 84.7 | 3.4 | − | Dysfunctional | 39 | 17p− |
| CLL 29 | I | 117.0 | 95.8 | 95.0 | 1.5 | + | ND | 9.5 | ND |
| CLL 31 | II | 68.8 | 94.7 | 90.8 | 4.7 | + | ND | 1.9 | 13q− |
| CLL 32 | 0 | 73.8 | 86.9 | 85.2 | 7.2 | + | ND | 2.4 | ND |
| CLL 40 | IV | 232.0 | 96.1 | 98.5 | 1.2 | − | ND | 97 | ND |
| Patient . | Rai stage . | WBC, 109/L . | Lymphocytes, % . | CD5+/ CD19+, %* . | CD3+, % . | IgVH† mutational status . | p53 status‡ . | CD38 (CD20+), % . | Chromosomal abnormalities§ . |
|---|---|---|---|---|---|---|---|---|---|
| CLL 01 | II | 113.0 | 90.0 | 88.3 | 4.4 | + | Functional | 81 | None |
| CLL 07 | I | 93.3 | 86.9 | 96.8 | 2.4 | + | Functional | 87 | 11q− |
| CLL 08 | 0 | 58.5 | 91.0 | 92.7 | 4.3 | + | ND | 24 | None |
| CLL 11 | II | 49.3 | 88.8 | 91.4 | 5.5 | − | Functional | 92 | 13q− |
| CLL 12 | I | 72.2 | 86.3 | 93.0 | 5.0 | + | Functional | 33 | None |
| CLL 16 | II | 80.1 | 73.7 | 92.0 | 6.0 | + | ND | 39 | 13q− |
| CLL 17 | II | 102.0 | 92.1 | 93.3 | 4.1 | − | ND | 47 | None |
| CLL 18 | 0 | 48.0 | 89.2 | 90.5 | 7.7 | + | ND | 29 | ND |
| CLL 20 | II | 61.4 | 89.0 | 95.0 | 4.0 | + | Functional | 20 | ND |
| CLL 21 | IV | 60.6 | 89.8 | 86.7 | 3.1 | − | ND | 24 | ND |
| CLL 22 | I | 60.1 | 94.4 | 81.7 | 6.5 | − | Dysfunctional | 44 | 17p− |
| CLL 23 | IV | 137.0 | 94.8 | 83.5 | 3.8 | + | Dysfunctional | 57 | 17p−;13q− |
| CLL 25 | I | 79.0 | 93.3 | 84.7 | 3.4 | − | Dysfunctional | 39 | 17p− |
| CLL 29 | I | 117.0 | 95.8 | 95.0 | 1.5 | + | ND | 9.5 | ND |
| CLL 31 | II | 68.8 | 94.7 | 90.8 | 4.7 | + | ND | 1.9 | 13q− |
| CLL 32 | 0 | 73.8 | 86.9 | 85.2 | 7.2 | + | ND | 2.4 | ND |
| CLL 40 | IV | 232.0 | 96.1 | 98.5 | 1.2 | − | ND | 97 | ND |
Patient data for the lymph node samples used in this study can be found in Smit et al.10
ND indicates not determined.
Percentage of cells positive for CD5 and CD19 surface expression.
Mutated IgVH gene (+) denotes more than 2% mutations compared with germline sequence.
p53 functional status was measured via radiation-induced RNA expression of p53 target genes Puma and Bax, or by p53 and p21 protein up-regulation via Western blot, as decribed in Mackus et al16 and Pettitt et al.25 Patient 25 had a so-called type A dysfunction.
As determined by FISH. Probes for 11q22.3 (ATM), 13q14 (D13S319), and 17p13 (TP53) were obtained from Abbott-Vysis. Samples with more than10% aberrant signals were considered abnormal.
RNA isolation and RT-MLPA
Reagents
The proteasome inhibitor bortezomib was obtained from Janssen-Cilag (Tilburg, The Netherlands). The γ-secretase inhibitor GSI-1, the Erk inhibitor PD-98 059, the NF-κB inhibitor Bay-11-7082, and the proteasome inhibitor MG132 were obtained from Calbiochem (Amsterdam, The Netherlands). Roscovitine and fludarabine (F-Ara-A) were purchased from Sigma-Aldrich. ABT-737 was obtained under a Material Transfer Agreement from Abbott (courtesy of Dr S. Rosenberg, Abbott Park, IL). The kinase inhibitors imatinib and dasatinib were from Novartis (Basel, Switzerland) and Bristol-Myers Squibb (New York, NY), respectively.
Analysis of apoptosis, Western blot, and antibodies
For apoptosis induction, cells at a density of 1.5 × 106/mL in culture medium were treated with 100 μM fludarabine (48 hours), 30 nM bortezomib, 25 μM roscovitine, or 5 μM GSI1 (24 hours), and stained with 200 nM MitoTracker Orange (Molecular Probes, Leiden, The Netherlands) for 30 minutes at 37°C and analyzed by FACS. Western blotting was performed as previously described.16 Cells were lysed in Laemmli sample buffer, and samples (10-30 μg protein) were separated by 13% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 10% gels for ERK). To screen for p53 functionality, cells were irradiated (5 Gy) and after overnight incubation tested for the expression of p53 and p21 by Western blot analysis as described before.25 Blots were probed with polyclonal anti–Mcl-1 (Pharmingen, BD Biosciences), monoclonal anti-Noxa (clone 114C307.1; Imgenex, San Diego, CA), monoclonal anti-Bim (clone 14A8; Chemicon, Temecula, CA), antiserum to β-actin (clone I-19; Santa Cruz Biotechnology, Santa Cruz, CA), polyclonal anti–Bcl-XL (BD Biosciences), polyclonal anti–Bcl-2 (Kordia, Leiden, The Netherlands), polyclonal anti–phospho-Erk (Cell Signaling), and polyclonal anti-Erk (Cell Signaling). Polyclonal antibodies against A1/Bfl-1 and Bid were a kind gift of Prof Dr J. Borst (The Netherlands Cancer Institute, Amsterdam, The Netherlands).
In vitro CD40 stimulation and cell lines
BCR-Abl–positive K562 cells and NIH3T3 fibroblasts were cultured in IMDM as described above for CLL cells. CD40-ligand (CD40L, CD154) was expressed on NIH3T3 fibroblasts, stably transfected with a plasmid encoding human CD40L. Fibroblasts were irradiated (30 Gy) and plated in culture-treated 6-well plates (6 × 105 cells/well). CLL cells were thawed and 5 × 106 cells per well were added to the adhered fibroblasts in 3 mL IMDM containing 10% FCS and incubated for 48 hours at 37°C. To test the effect of c-Abl kinase inhibitors imatinib and dasatinib, and the effect of Erk-inhibitor PD-58 059, CLL cells were pretreated with 80 μM imatinib or dasatinib, or 50 μM PD-58 059 for 30 minutes. After preincubation CLL cells were stimulated for 48 hours at 37°C with CD40L with or without 30 μM imatinib or dasatinib or 50 μM PD-58 059. In the case of dasatinib, also other regimens and concentrations were used, where CLL cells were first cocultured for 48 hours with CD40L-expressing or control 3T3 fibroblasts, detached and washed, and subsequently incubated in medium for an additional 48 hours in the presence of varying dasatinib concentrations (30 nM-30 μM), followed by testing sensitivity to cytotoxic drugs, as described under “Analysis of apoptosis, Western blot, and antibodies.”
Results
Prolonged CD40 stimulation of CLL cells results in broad drug resistance, which is independent of ERK-mediated decrease in Bim levels
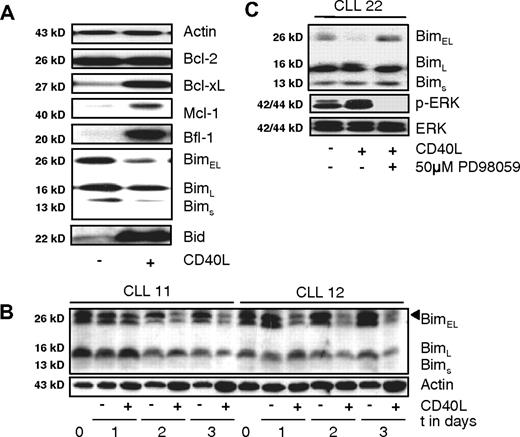
In vitro stimulation via CD40 renders CLL cells resistant to fludarabine and induces expression of various antiapoptotic proteins such as Bcl-Xl and A1/Bfl-1 via de novo transcription.12,13,26 In addition to previously described transcriptional effects of prolonged CD40 triggering, several novel effects on protein levels of various apoptosis regulators were observed. In particular, the Bim-EL splice variant decreased whereas Mcl-1 levels increased (Figure 1A).
Antiapoptotic changes in CLL cells upon CD40 stimulation include ERK-mediated decrease in Bim levels. (A) Changes in expression of apoptosis regulators upon 48 hours of CD40 triggering were monitored by Western blot for the indicated proteins. Results are from a representative CLL sample from more than 10 patients studied. Equal protein loading was confirmed by staining for actin as loading control. (B) Time course of Bim-EL decrease monitored in 2 CLL samples. Shown are samples taken on consecutive days of coculture in absence or presence of CD40 stimulation. In sample 11 a decrease in Bim-EL can be observed on day 2, and in sample 12 on day 1. Position of Bim-EL is indicated by a triangle, the faster migrating species is probably the Bimα1 splice variant. (C) Effects of ERK inhibition on Bim levels and phosphorylated ERK. CLL cells were stimulated with CD40 in the presence of ERK inhibitor as indicated, and lysates were probed for Bim protein, phosphorylated and total ERK levels.
Antiapoptotic changes in CLL cells upon CD40 stimulation include ERK-mediated decrease in Bim levels. (A) Changes in expression of apoptosis regulators upon 48 hours of CD40 triggering were monitored by Western blot for the indicated proteins. Results are from a representative CLL sample from more than 10 patients studied. Equal protein loading was confirmed by staining for actin as loading control. (B) Time course of Bim-EL decrease monitored in 2 CLL samples. Shown are samples taken on consecutive days of coculture in absence or presence of CD40 stimulation. In sample 11 a decrease in Bim-EL can be observed on day 2, and in sample 12 on day 1. Position of Bim-EL is indicated by a triangle, the faster migrating species is probably the Bimα1 splice variant. (C) Effects of ERK inhibition on Bim levels and phosphorylated ERK. CLL cells were stimulated with CD40 in the presence of ERK inhibitor as indicated, and lysates were probed for Bim protein, phosphorylated and total ERK levels.
Since it is known that ERK signaling can affect Bim-EL protein levels in model systems21,27 this aspect was investigated further. Over the course of several days of CD40 stimulation, a significant reduction in Bim-EL protein levels occurred, although Bim mRNA levels remained constant.10 In Figure 1B, the larger 2 Bim species represent Bim-EL and most presumably a splice variant Bimα1,28 which became visible in certain samples upon prolonged migration in SDS-PAGE. Under the experimental conditions applied, a short-lived phosphorylated form of Bim (p-Bim) is probably also present,21 but in our hands this form of Bim could not be observed in primary samples either with the antibody used here, or with commercial antibodies specifically generated against p-Bim. The activation status of ERK upon CD40 triggering was increased, and addition of the specific ERK inhibitor PD-98 059 during CD40 stimulation prevented the reduction of Bim-EL (Figure 1C). Addition of the proteasome inhibitor MG132 after CD40 stimulation demonstrated that Bim-EL levels were controlled via increased protein turnover, confirming previous reports21,22,29 (data not shown).
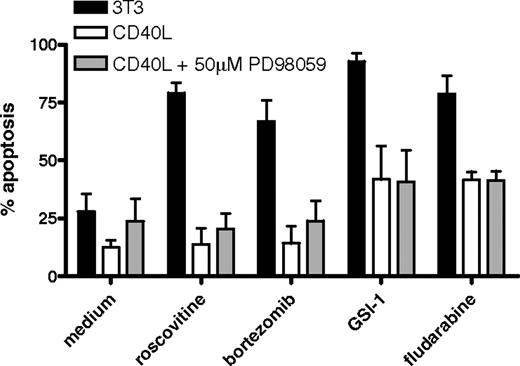
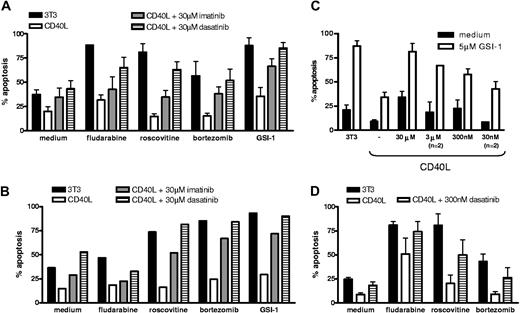
Next, CLL cells triggered via CD40 in the absence or presence of ERK inhibition were investigated for sensitivity to drugs that are in current clinical use or in preclinical development. As can be seen in Figure 2, prolonged CD40 stimulation rendered the cells resistant to fludarabine, as observed before,12,13 the proteasome inhibitor bor-tezomib, and the cyclin-dependent kinase inhibitor roscovitine. In addition, the γ-secretase inhibitor GSI-1 was included, which is considered to be an inhibitor of Notch signaling.30 We have recently observed that GSI-1 is in fact an inhibitor of the proteasome and a potent inducer of apoptosis in CLL (D.Y.H.H., Heike Schmidlin, M.H.J.v.O., E.E., manuscript submitted July 3, 2008). CD40 triggering also rendered CLL cells resistant to GSI-1. For multiple CLL isolates tested, addition of ERK inhibitors did not alleviate the broad drug resistance afforded via prolonged CD40 stimulation (Figure 2). Together these data indicate that although CD40 signaling activates ERK and thereby causes a decline in Bim-EL levels, this is not the cause for the observed broad drug resistance.
Broad drug resistance of CLL cells upon CD40 stimulation is not prevented by ERK inhibition. CLL cells were cocultured with control 3T3 (control) or CD40L-expressing cells for 48 hours, in the presence of ERK inhibitor PD98059 as indicated. After detachment and washing, cells were incubated with the indicated drugs as described in detail in “Methods,” and analyzed for apoptosis by MitoTracker staining after 24 hours (roscovitine, bortezomib, and GSI-1) or 48 hours (fludarabine). Cells cultured on 3T3 cells (■) are sensitive to all drugs, but CD40 stimulation (□) confers broad drug resistance and this is maintained when ERK is inhibited ( ). The data shown for untreated samples (medium) were measured at 24 hours. Apoptosis levels of medium samples at 48 hours were comparable.
). The data shown for untreated samples (medium) were measured at 24 hours. Apoptosis levels of medium samples at 48 hours were comparable.
Broad drug resistance of CLL cells upon CD40 stimulation is not prevented by ERK inhibition. CLL cells were cocultured with control 3T3 (control) or CD40L-expressing cells for 48 hours, in the presence of ERK inhibitor PD98059 as indicated. After detachment and washing, cells were incubated with the indicated drugs as described in detail in “Methods,” and analyzed for apoptosis by MitoTracker staining after 24 hours (roscovitine, bortezomib, and GSI-1) or 48 hours (fludarabine). Cells cultured on 3T3 cells (■) are sensitive to all drugs, but CD40 stimulation (□) confers broad drug resistance and this is maintained when ERK is inhibited ( ). The data shown for untreated samples (medium) were measured at 24 hours. Apoptosis levels of medium samples at 48 hours were comparable.
). The data shown for untreated samples (medium) were measured at 24 hours. Apoptosis levels of medium samples at 48 hours were comparable.
c-Abl inhibors prevent the antiapoptotic protein profile of CD40-treated CLL cells
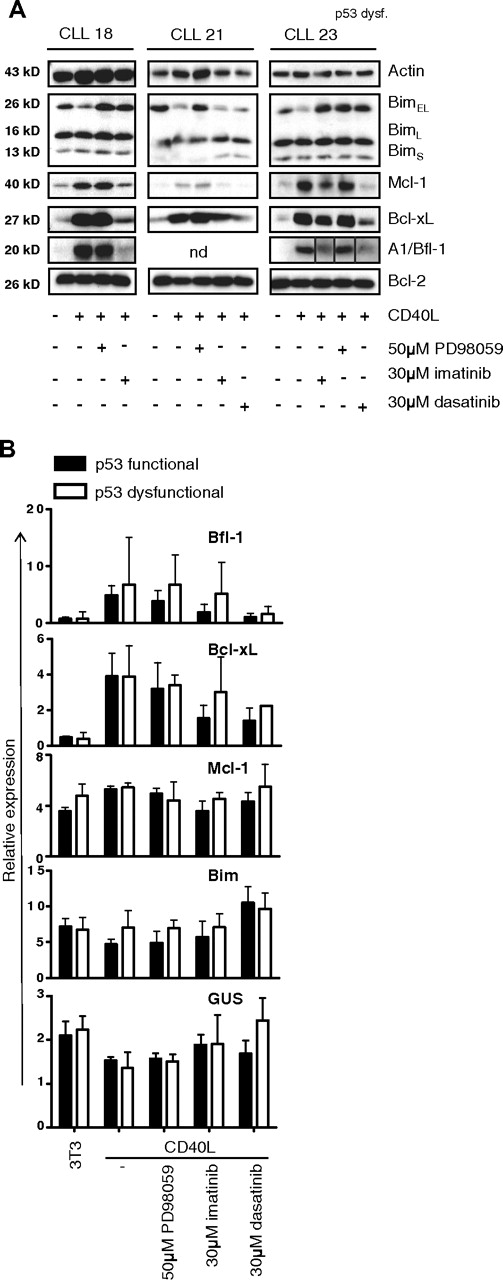
Another aspect of prolonged CD40 triggering of CLL cells was an increase in Mcl-1 protein (Figure 1A) which was, similar to the changes in Bim, independent from increased transcription. Mcl-1 has recently been recognized as a promising target for drugs,31 and has been implicated in antiapoptotic signaling via BCR-Abl in chronic myeloid leukemia.32-34 Furthermore, other antiapoptotic changes in our in vitro CD40-CLL system, such as increased Bcl-XL and decreased Bim, have also been implicated in BCR-Abl signaling.32,35-37 Lastly, it was recently reported that c-Abl protein expression correlates positively with tumor burden and disease stage in CLL.38 Therefore, we next tested the c-Abl inhibitor STI-571/gleevec/imatinib as a potential suppressor of CD40-mediated prosurvival effects in CLL cells. In Figure 3 it can be seen that imatinib caused a clear reversal of almost all effects of CD40 stimulation regarding Bcl-XL, Mcl-1, A1/Bfl-1, and Bim levels (left panel). This was also observed for the second generation Abl inhibitor sprycel/dasatinib (middle panel). This compound has a higher specific activity toward c-Abl, but is also less specific for Abl kinase and targets other kinases such as Btk, Lyn, and Tec.23,39 The effects of imatinib and dasatinib with respect to reversing the CD40 effects on prosurvival parameters were also observed in CLL cells with a dysfunction in the p53 pathway (Figure 3 right panel).
Antiapoptotic gene and protein profile of CLL induced by CD40 stimulation is reversed by kinase inhibitors imatinib and dasatinib. (A) CLL cells were cocultured with control 3T3 or CD40L-expressing cells for 48 hours, in the presence of PD98059, imatinib, or dasatinib as indicated. Lysates were probed for Bim, Mcl-1, Bcl-XL, A1/Bfl-1, and Bcl-2 as indicated and actin as loading control. Shown are representative examples of 2 CLL samples with wild-type (WT) p53 function (left and middle panels), and 1 CLL with p53 dysfunction (right panel). Note different order of samples in this panel and that the lanes of the A1/Bfl1 blot have been repositioned to match the other blots from the same experiment. Vertical lines have been inserted to mark the adjusted lanes. The up-regulation of Mcl-1, Bcl-XL, and A1/Bfl-1 is not affected by ERK inhibition, but prevented by imatinib or dasatinib, irrespective of p53 functionality. (B) RNA was collected from CLL cells stimulated for 48 hours with CD40 and inhibitors as indicated, and assayed for expression of 34 apoptosis genes by MLPA. Shown are averaged relative expression levels plus or minus SD (in percentage of total normalized signal) of selected genes in samples from p53 WT (n = 4) and p53 dysfunctional (n = 3) CLL cells. The CD40-mediated positive effects on transcription of A1/Bfl-1 and Bcl-XL are reversed by Abl kinase inhibitors. Examples of genes that are not significantly affected at the transciptional level are Mcl-1, Bim, and GUS (β-glucuronidase, a housekeeping gene).
Antiapoptotic gene and protein profile of CLL induced by CD40 stimulation is reversed by kinase inhibitors imatinib and dasatinib. (A) CLL cells were cocultured with control 3T3 or CD40L-expressing cells for 48 hours, in the presence of PD98059, imatinib, or dasatinib as indicated. Lysates were probed for Bim, Mcl-1, Bcl-XL, A1/Bfl-1, and Bcl-2 as indicated and actin as loading control. Shown are representative examples of 2 CLL samples with wild-type (WT) p53 function (left and middle panels), and 1 CLL with p53 dysfunction (right panel). Note different order of samples in this panel and that the lanes of the A1/Bfl1 blot have been repositioned to match the other blots from the same experiment. Vertical lines have been inserted to mark the adjusted lanes. The up-regulation of Mcl-1, Bcl-XL, and A1/Bfl-1 is not affected by ERK inhibition, but prevented by imatinib or dasatinib, irrespective of p53 functionality. (B) RNA was collected from CLL cells stimulated for 48 hours with CD40 and inhibitors as indicated, and assayed for expression of 34 apoptosis genes by MLPA. Shown are averaged relative expression levels plus or minus SD (in percentage of total normalized signal) of selected genes in samples from p53 WT (n = 4) and p53 dysfunctional (n = 3) CLL cells. The CD40-mediated positive effects on transcription of A1/Bfl-1 and Bcl-XL are reversed by Abl kinase inhibitors. Examples of genes that are not significantly affected at the transciptional level are Mcl-1, Bim, and GUS (β-glucuronidase, a housekeeping gene).
The various kinase inhibitors were also monitored for their effects on transcription using a multiplex assay able to quantify expression of 34 apoptosis regulatory genes. As described previously, prolonged in vitro CD40 stimulation of CLL cells induces transcription of Bcl-XL and A1/Bfl-1, as well as a reduction in Noxa.10,13 For the ERK inhibitor PD-98 059, no effects on transcription of these genes were found. In contrast, the c-Abl inhibitors prevented up-regulation of Bcl-XL and A1/Bfl-1 transcripts, whereas, for example, Mcl-1 and Bim transcripts were hardly affected by these drugs (Figure 3B filled bars), although they did display changes at the protein level (Figure 3A). The effects of the Abl kinase inhibitors on Bcl-XL and A1/Bfl-1 were similar to those observed when CLL cells were exposed to NF-κB inhibitor BAY-117082 during stimulation via CD40 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The inhibitory effects of especially dasatinib on Bcl-Xl and A1/Bfl-1 transcription were also detected in cells with a dysfunctional p53 response (Figure 3B open bars). In these cases, the effects of imatinib on CD40-induced gene transcription were limited, suggesting that perhaps the suppressive effects of imatinib may require p53 function. The complete dataset for all genes interrogated by the MLPA probe set is represented in Figure S2. Together these data demonstrate that imatinib/dasatinib have a clear impact on signaling pathways leading to gene transcription such as NF-κB, and also on mechanisms controlling protein turnover of Mcl-1 and Bim.
Contribution to drug resistance of prosurvival proteins probed by ABT-737
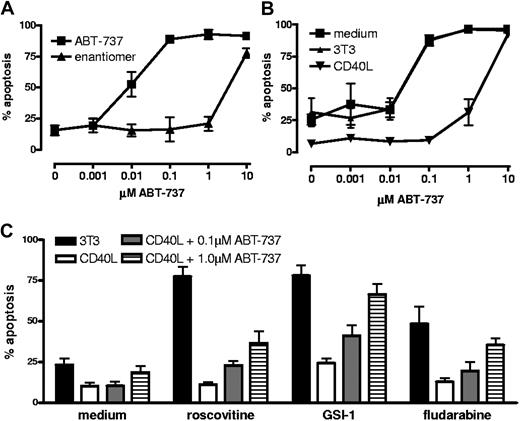
Antiapoptotic Bcl-2 family members can be counteracted by BH3 mimetics such as ABT-737, a widely studied compound in preclinical development.40 ABT-737 is very effective against Bcl-2 and Bcl-XL, but does not bind to Mcl-1 or A1/Bfl-1.31,41 As reported before,42 CLL cells are quite sensitive to ABT-737, but upon stimulation with CD40 this is reduced approximately 100-fold (Figure 4A,B). We tested whether sublethal doses of ABT-737 could synergize with other drugs in this setting. There was a slight increase in apoptosis of CD40-stimulated cells when 0.1 μM ABT-737 was combined with various other drugs. This was further restored to levels observed in medium or control cultures with 3T3 cells by using 1 μM ABT-737. In Figure 4C the averaged data from 4 patients with CLL are shown. Individual sample responses to ABT-737 showed divergent patterns, with some patient's cells displaying full reversal of drug sensitivity at 1.0 μm ABT-737 for all drugs tested, while others displayed different patterns depending on the drug tested (Figure S3). This seemed consistent with the clear patient-to-patient variation in the degree of up-regulation of Mcl-1 and A1/Bfl-1 (Figure 3A). These results indicate that the contribution of Bcl-2 and Bcl-XL to the observed drug resistance in this in vitro model is substantial, but could generally be counteracted by ABT-737. The efficiency and effective dose with which ABT-737 acts differs per patient sample and this probably correlates with the degree of increase in Mcl-1, and possibly A1/Bfl-1, obtained with CD40 stimulation.
Contribution of Mcl-1 to drug resistance probed by ABT-737. (A) CLL cells were treated immediately after thawing with the indicated concentrations of ABT-737 or the inactive enantiomer. After 24 hours, apoptosis was measured by Mitotracker staining. (B) CLL cells were cultured for 2 days in medium, with 3T3 control cells or with 3T40L cells before treatment with ABT-737 as above. Data in panels A and B represent averages plus or minus SD from 3 different CLL samples. (C) Sublethal doses of ABT-737 after CD40 stimulation as determined in panel B (0.1 μM and 1.0 μM) were combined with various other drugs as indicated to test synergy in reversal of drug resistance. Data are averages plus or minus SD from 5 (0.1 μM) or 4 (1 μM) patient samples, tested in 3 independent experiments
Contribution of Mcl-1 to drug resistance probed by ABT-737. (A) CLL cells were treated immediately after thawing with the indicated concentrations of ABT-737 or the inactive enantiomer. After 24 hours, apoptosis was measured by Mitotracker staining. (B) CLL cells were cultured for 2 days in medium, with 3T3 control cells or with 3T40L cells before treatment with ABT-737 as above. Data in panels A and B represent averages plus or minus SD from 3 different CLL samples. (C) Sublethal doses of ABT-737 after CD40 stimulation as determined in panel B (0.1 μM and 1.0 μM) were combined with various other drugs as indicated to test synergy in reversal of drug resistance. Data are averages plus or minus SD from 5 (0.1 μM) or 4 (1 μM) patient samples, tested in 3 independent experiments
c-Abl kinase inhibitors prevent drug resistance of CD40-treated CLL cells
In a similar fashion as for ABT-737, the effect of c-Abl kinase inhibitors on the drug resistance afforded by CD40 triggering was measured. The apoptosis-inducing effects of the Abl inhibitors themselves on control samples cocultured with 3T3 cells and CD40L-expressing cells were minimal (Figure 5A medium samples). Only at high levels and upon prolonged exposure did imatinib and dasatinib induce significant apoptosis in CLL cells, in contrast to, for example, K562 cells, which are very sensitive due to their dependence on the BCR-Abl fusion protein for survival (Figure S4). Remarkably, however, imatinib and especially dasatinib prevented the resistance toward various drugs normally observed upon CD40 treatment of CLL cells. This appeared true for CLL samples with mutated as well as unmutated IgVH gene sequences (both n = 2). The sensitizing effect of these inhibitors was also seen in CLL cells with a dysfunctional p53 pathway (Figure 5B). Especially the cytotoxic effect of proteasome inhibitors (bortezomib and GSI-1) was potentiated by treatment of CLL cells with c-Abl inhibitors during CD40 exposure. In general, the effects of dasatinib were stronger than those of imatinib at the concentrations used (30 μM), as was also observed for the effects on protein levels (Figure 3). Since dasatinib has a higher specific activity toward its target kinases than imatinib23,43 we also tested its effects over a lower range of concentrations. The capacity of dasatinib to modulate the drug sensitivity of CD40-treated CLL cells could also be observed at substantially lower concentrations (30 nM-3 μM). This is demonstrated in Figure 5C for the results obtained with GSI-1, which was in general the strongest inducer of apoptosis in CLL cells among the drugs tested.
Drug resistance of CD40-stimulated CLL cells is reversed by c-Abl kinase inhibitors. (A) CLL samples (n = 4) were cultured on 3T3 (control) or CD40L-expressing cells in the presence of the indicated inhibitors for 48 hours, and after detachment and washing cultured for 24 hours in medium or with the cytotoxic drugs. Average results for apoptosis measured via Mitotracker staining are shown. (B) The same as in panel A for an experiment with p53 dysfunctional cells. Data are representative for 3 similar experiments performed; the variation among samples in particular for background apoptosis in the absence of external stimuli precluded averaging. (C) A similar experiment as in panel A was performed with decreasing concentrations of dasatinib as indicated. Drug susceptibility was assessed by incubation with 5 μM GSI-1 for 24 hours. Results represent averages of 4 experiments or 2 where indicated. At 3 nM there was no effect of dasatinib detectable (not shown). (D) Sequential CD40 stimulation followed by incubation with c-Abl kinase inhibitors. CLL cells were cocultured with 3T3 cells expressing CD40L for 48 hours, detached and washed before addition of dasatinib (300 nM) for an additional 48 hours, and were then tested for drug susceptibility. Results represent average data of 3 experiments.
Drug resistance of CD40-stimulated CLL cells is reversed by c-Abl kinase inhibitors. (A) CLL samples (n = 4) were cultured on 3T3 (control) or CD40L-expressing cells in the presence of the indicated inhibitors for 48 hours, and after detachment and washing cultured for 24 hours in medium or with the cytotoxic drugs. Average results for apoptosis measured via Mitotracker staining are shown. (B) The same as in panel A for an experiment with p53 dysfunctional cells. Data are representative for 3 similar experiments performed; the variation among samples in particular for background apoptosis in the absence of external stimuli precluded averaging. (C) A similar experiment as in panel A was performed with decreasing concentrations of dasatinib as indicated. Drug susceptibility was assessed by incubation with 5 μM GSI-1 for 24 hours. Results represent averages of 4 experiments or 2 where indicated. At 3 nM there was no effect of dasatinib detectable (not shown). (D) Sequential CD40 stimulation followed by incubation with c-Abl kinase inhibitors. CLL cells were cocultured with 3T3 cells expressing CD40L for 48 hours, detached and washed before addition of dasatinib (300 nM) for an additional 48 hours, and were then tested for drug susceptibility. Results represent average data of 3 experiments.
The results thus far were obtained with simultaneous administration of CD40 signals and kinase inhibitors. To better reflect the actual situation of LN CLL cells already exposed to a protective environment, isolated PB CLL cells were first stimulated via CD40 for 48 hours, followed by separate addition of dasatinib (0.3 μM) and drug-sensitivity tests. Also in this set-up, a reversal of resistance toward various drugs (fludarabine, bortezomib, roscovitine) could be observed (Figure 5D). Thus, dasatinib has a clear capacity to interfere with the protective effects afforded by prolonged CD40 stimulation.
Similar apoptosis protein signature in ex vivo LN samples as upon vitro CD40 triggering
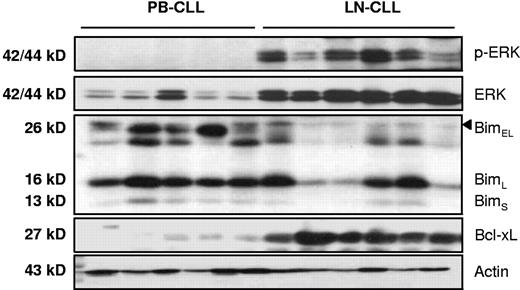
To relate the effects of in vitro CD40 stimulation with the in vivo situation, samples from CLL lymph nodes were lysed directly in SDS-containing sample buffer and probed for the presence of proteins involved in apoptosis regulation. As observed before, a clear increase of Bcl-XL protein was present in LN samples compared with peripheral blood (PB) samples.10 This was also found for Mcl-110 and A1/Bfl-1 (data not shown). Regarding the expression levels of other signature proteins involved in CD40-mediated antiapoptosis pathways, a strong increase in both total and phosphorylated ERK was found, concomitant with decreased levels of Bim-EL (Figure 6). These findings indicate that in CLL lymph nodes similar survival pathways are operational as those that can be induced in peripheral blood CLL cells by prolonged in vitro CD40 stimulation.
Antiapoptotic protein signature in CLL lymph nodes. Protein lysates obtained from peripheral blood (PB, n = 5) and lymph node (LN, n = 6) were probed for phosphorylated-ERK, total ERK, Bim, and actin as indicated. The expression of these proteins in ex vivo LN was similar to changes observed upon in vitro stimulation of PB CLL cells with CD40.
Antiapoptotic protein signature in CLL lymph nodes. Protein lysates obtained from peripheral blood (PB, n = 5) and lymph node (LN, n = 6) were probed for phosphorylated-ERK, total ERK, Bim, and actin as indicated. The expression of these proteins in ex vivo LN was similar to changes observed upon in vitro stimulation of PB CLL cells with CD40.
Discussion
Previous reports have described effects of inhibitors of BCR-Abl kinase on single antiapoptosis proteins (predominantly Mcl-1, Bcl-xl, or Bim) in CML or model cell lines.35-37 This study provides an overview on the effects of c-Abl inhibitors on all Bcl-2 members in the context of CD40 signaling in CLL cells. The rationale for the present study was 2-fold. First is the growing concept that CLL is a dynamic disease, with proliferation centers in LN and possibly also BM. These protective niches, where cells are prone to be more drug resistant, are presumably the source of relapsing clones. Second is the potential of novel drugs such as kinase inhibitors to target prosurvival signaling pathways to which malignant cells have become addicted.
We have observed that our in vitro CLL culture model setting provides strong and probably supraphysiologic CD40 signals, with long-lasting protective effects which continue after detachment of CLL cells from CD40L cells (data not shown). Nevertheless, comparison between LN samples and PB CLL cells stimulated in vitro via CD40 indicated the presence of a comparable prosurvival signature as implied by ERK activation and Bim-EL levels. Previously, we have shown that in LN samples increased levels of Bcl-XL and Mcl-1 are also detectable.10 Together, the available data indicate that the prosurvival signature triggered via CD40 stimulation in vitro is also found in CLL lymph nodes, and imply that our experimental data hold promise for extrapolation toward a therapeutic setting.
With respect to posttranscriptional effects of CD40 stimulation on CLL cells, both Bim and Mcl-1 proteins are known targets for phosphorylation and subsequent increased proteasomal degradation. Cytokine withdrawal in murine cell lines causes decreased PI3K-Akt/PKB signaling to activate GSK3, which in turn phosporylates Mcl-1, thus marking it for proteasomal degradation.44 In the case of CLL cells, our data indicate that upon CD40 stimulation PKB phosphorylation was undetectable, the PI3 kinase inhibitor LY294002 did not trigger apoptosis, and the rate of Mcl-1 protein turnover was not changed (A.J., E.E., unpublished observations, March 2008). Since Mcl-1 transcription in CLL cells was also not affected by CD40, this suggests that the increase in Mcl-1 protein is possibly controlled at the level of translation by a non–PKB-dependent mechanism. Recent evidence from other experimental systems indeed points at translational repression of Mcl-1 via eIF initiation factors as yet another point of regulation.45,46 If this system is operational under our experimental conditions and whether it may be connected with the other recently described pathway implicating antigen receptor/PI3-K/PKB signaling in affecting Mcl-1 levels47 remains to be determined. In contrast to the situation in AML cells,41 in primary CLL cells the ERK pathway seems not responsible for increased Mcl-1 protein, as the ERK inhibitor PD-98 059 did not block its increase, and did not affect drug susceptibility (Figures 2 and 3). Whether or not increased Mcl-1 plays an important role in vivo in survival of CLL in lymph nodes seems an important issue with respect to therapeutic application of ABT-737. Our data and those of others31,41 indicate that variations in Mcl-1 and possibly also A1/Bfl-1 levels will determine the effective dose of ABT-737 both as a single agent and in drug combinations. Of note, the combination of ABT-737 with roscovitine, which should counteract Bcl-2, Bcl-XL, and Mcl-1,31 was not effective in all patients (Figure S4). This suggests that either roscovitine is unable to reduce Mcl-1 in this setting, or that perhaps in these samples A1/Bfl-1 is a dominant factor. Our observations on increased Bim-EL turnover are in accord with an established pathway of ERK-mediated phosphorylation and proteasomal degradation.21,22,29 To our knowledge, this is the first example of this pathway operating in primary tumor cells upon CD40 stimulation, and in CLL LN samples.
In our experience, neither imatinib nor dasatinib are efficient inducers of apoptosis as single agents, in contrast to their effects on K562 cells, which depend for survival on the BCR-Abl fusion oncogene (Figure S3). In a recent study, considerable variation in apoptosis susceptibility in untreated and dasatinib-treated peripheral blood samples was found using 5 μM dasatinib, and the response was correlated with IgVH mutation and ZAP70 status.48 This and other studies performed to date agree that in CLL cells from peripheral blood, dasatinib has a strong synergistic effect in combination with p53 pathway–dependent and –independent agents.48-50
Transcriptional effects of imatinib and dasatinib on Bcl-XL and A1/Bfl-1 were similar to those of inhibitors of NF-kB. We noted that the effects of the Abl inihitors on reversing ERK phosphorylation status, and the corresponding changes in Bim levels varied among patients, without apparent correlation with prognostic factors such as mutation or p53 status (data not shown and Figure 3). These signaling pathways are affected/reversed by imatinib and dasatinib although the actual target(s) remains unknown. Recent analyses of the spectrum of kinase targets of these compounds points to various candidates involved in T- and/or B-cell activation such as Src kinases including Lck and Fyn, Btk, and Tec kinase.23,39 The spectrum of non-Abl kinases targeted by dasatinib is in fact quite broad (> 20 kinases), and an immunosuppressive effect was predicted23 and recently confirmed for T cells.51 Our preliminary analyses do not show a similar inhibitory effect of dasatinib on in vitro B-cell proliferation, however (A.J., E.E., unpublished observation, November 2007). From the kinases targeted by dasatinib no obvious candidate(s) for exclusive participation in the CD40 pathway is apparent, although the Ser/Thr kinase p38α and upstream MAP kinases appear likely as participants. A clue for the participation of Btk or Tec kinases comes from a recent report that their expression level is regulated via NF-κB in a positive feedback-loop. This loop can be interrupted by proteasome inhibitors,52 which fits with our observation that the combination of bortezomib or GSI-1 with dasatinib has the strongest effect on apoptosis of CD40-stimulated CLL cells (Figure 5A,B).
Obviously, c-Abl kinase itself may very well be involved, and there is evidence that levels of c-Abl protein expression correlate positively with tumor burden and disease stage in CLL.53 Another study reported that c-Abl becomes active upon CD40 triggering and then induces p73.54 This signaling route is predicted to bypass p53 and may therefore be therapeutically relevant. Both these studies used imatinib and/or introduction of recombinant c-Abl, so they cannot provide definitive evidence of endogenous c-Abl kinase activity in CLL. The majority of studies on activity have been done with the BCR-Abl–positive cell line K562 or primary CML samples where expression levels of the oncogenic fusion protein are augmented. Our preliminary efforts to detect active endogenous c-Abl either in unstimulated, CD40-triggered, or LN CLL cells by Western blotting with commercial antibodies were inconsistent.
At present, 2 independent mechanisms are attributed to the development of chemoresistance in CLL. The first is a shift in the balance between pro- and antiapoptotic regulators, and both Mcl-155 and Bfl-1/A156 have been associated with resistance to chemotherapy. Significantly, these hallmarks are very similar to the CD40-activated CLL phenotype we use as a model. The second mechanism is based on acquired mutations resulting in a dysfunctional p53 response.3 A recent phase 2 evaluation of dasatinib as single agent in relapsed and refractory CLL showed limited effects, but in good correlation with our data a reduction of lymph node size was observed in a major fraction of patients.57 Our data indicate that c-Abl inhibitors, notably dasatinib, overcome the protective profile within the micro-environment resulting in susceptibility to p53 pathway–dependent drugs (fludarabine) as well as to p53-independent agents (roscovitine, bortezomib, ABT-737). Thus, from a clinical perspective it may be more effective to apply combination strategies of dasatinib with other drugs. Our data provide a rationale to combine dasatinib both with purine-analogues but also with drug regimens that do not exclusively rely on p53 function for efficacy.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr M. Kramer, Dr S. Wittebol (Department of Internal Medicine, Meander Medical Center, Amersfoort), and Dr J. Baars (Department of Internal Medicine, The Netherlands Cancer Institute, Amsterdam) for including patients with CLL in this study, and René van Lier for his insightful comments and critical reading of the manuscript.
This work was supported by the Dutch Cancer Foundation (DCF) grant no. UVA2004-3039. A.P.K. is supported by a Veni grant from ZonMw (The Netherlands Organization for Health Research and Development).
Authorship
Contribution: D.Y.H.H. and A.J. performed research and wrote the paper; C.J.v.N. contributed patient samples and analyzed data; M.H.J.v.O. supervised research and analyzed data; A.P.K. analyzed data and wrote the paper; and E.E. supervised research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eric Eldering, Department of Experimental Immunology, Academic Medical Center, Room K0-144, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: e.eldering@amc.uva.nl.