Abstract
Interleukin-21 (IL-21) is a recently described immunoregulatory cytokine. It has been identified as a very potent immunotherapeutic agent in several cancer types in animal models, and clinical studies are ongoing. IL-21 belongs to the type I cytokine family of which other members, ie, IL-2, IL-15, and IL-4, have been shown to exert activities on vascular endothelial cells (ECs). We hypothesized that IL-21, in addition to inducing the antitumor immune response, also inhibits tumor angiogenesis. In vitro experiments showed a decrease of proliferation and sprouting of activated ECs after IL-21 treatment. We found that the IL-21 receptor is expressed on vascular ECs. Furthermore, in vivo studies in the chorioallantoic membrane of the chick embryo and in mouse tumors demonstrated that IL-21 treatment disturbs vessel architecture and negatively affects vessel outgrowth. Our results also confirm the earlier suggested angiostatic potential of IL-2 in vitro and in vivo. The angiostatic effect of IL-21 is confirmed by the decrease in expression of angiogenesis-related genes. Interestingly, IL-21 treatment of ECs leads to a decrease of Stat3 phosphorylation. Our research shows that IL-21 is a very powerful antitumor compound that combines the induction of an effective antitumor immune response with inhibition of tumor angiogenesis.
Introduction
Interleukin (IL)-21, the most recently described member of the common γ-chain cytokine family, is found to be a potent immunoregulatory cytokine.1 This cytokine is produced by activated CD4+ T cells and targets several immune cells,2-5 including NK cells,3,4,6-8 dendritic cells, cytotoxic T-lymphocytes,3,4,9 as well as B cells.10-13 Structurally, IL-21 is considered to be a member of the type I cytokine family, which includes IL-2, IL-4, and IL-15.2,14-16 The receptors for all these type I cytokines contain the common γ-chain.2,14-16 In leukocytes, type I cytokines activate the Janus family tyrosine kinases, JAK1 and JAK3,2 which in turn regulate activation of signal transducer and activator of transcription 1 (Stat1), Stat3, Stat5a, and Stat5b.2,17-19 IL-2 and IL-15 mainly activate STAT5a and STAT5b, whereas IL-4 primarily activates Stat6.2 IL-21 is demonstrated to activate Stat1, Stat3, Stat5a, and Stat5b. The activation of Stat5a and Stat5b by IL-21 is weak and transient, whereas the activation of Stat3 is the most sustained. Stat3 appears to be the most important STAT protein for IL-21 signaling.20 This activation of Stats is cell type–specific. Interestingly, in endothelial cells (ECs), Stat3 phosphorylation has been associated with induction of angiogenesis.21,22 Recent studies suggest that IL-21 can be used as an immunomodulatory compound,23,24 and its use in the clinical setting is under way.25
The process of new blood vessel formation or angiogenesis is essential for tumor growth and metastasis, as it is critical for supply of oxygen and nutrients. Inhibition of angiogenesis is therefore a promising strategy for treatment of cancer.26,27 Several antiangiogenesis compounds have been developed and tested based on their ability to inhibit proangiogenic growth factors, such as vascular endothelial growth factor (VEGF) (SU6668, SU11248, and bevacizumab)28-30 or intervention in signal transduction in endothelial cells directly (TNP-470, endostatin, and anginex).31-34 Members of the type I-cytokine family, ie, IL-2, IL-4, are suggested to affect the formation of new blood vessels,35-37 but their ability to block angiogenesis has not been carefully tested. In analogy to IL-2 and IL-4, we hypothesized that IL-21 inhibits angiogenesis and thus mediates antitumor efficacy partly because of its effects on endothelial growth and sprouting. These properties along with its ability to regulate various immune cells endow IL-21 with the potent ability to promote tumor regression.
To test this notion, we conducted experiments to demonstrate the effects of IL-21 on ECs and compared it with the effects of IL-2. Our results demonstrate that IL-21 mediates angiostatic properties, as determined by in vitro and in vivo assays. This contributes to antitumor activity of IL-21 in vivo. These results suggest that IL-21 is a very powerful antitumor compound, having the ability to induce an effective antitumor immune response and simultaneously inhibit tumor angiogenesis. This is the first demonstration of the antiangiogenic property of IL-21, which may have considerable implications for the use of IL-21 in future clinical trials.
Methods
Cell culture and reagents
The murine EC cell line SVEC4-10 and TME were purchased from ATCC (Manassas, VA) and cultured in Dulbecco modified Eagle medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS; HyClone Perbio Science, Erembodegem-Aalst, Belgium), 2 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), and 2 mM l-glutamine (Invitrogen) in 0.2% gelatin (Sigma-Aldrich, St Louis, MO) coated tissue culture flasks (Corning Life Sciences, Acton, MA). The cells were subcultured 1:10. Human umbilical vein–derived ECs (HUVECs) were cultured as described previously.31 Angiogenic stimulation of the ECs was induced by 10 ng/mL basic fibroblast growth factor (bFGF) and/or 40 ng/mL VEGF (PeproTech, Rocky Hill, NJ). Primary mouse ECs were isolated as described earlier.38 Normal heart tissues derived from C57Bl/6 mice were mechanically and enzymatically digested. The obtained single cell suspensions were allowed to adhere to gelatin coated tissue culture flasks (Corning Life Sciences). The remaining adherent cell population was stained for the CD31 marker and separated from other cells by sorting (FACSAria; BD Biosciences, Alphen a/d Rijn, The Netherlands). Cells were cultured in DMEM containing 20% FCS.
Primary human tumor and normal ECs were isolated from fresh tissues as described previously.39 Briefly, colorectal carcinoma tissues and distant normal colon tissue of the same patients were obtained directly after surgery, minced, and digested to create a single-cell suspension. ECs were immunolabeled with a combination of anti-CD31 (clones JC/70A; Dako North America, Carpinteria, CA; EN4; Monosan, Sanbio, Am Uden, The Netherlands) and anti-CD34 (clone Qbend10; Novocastra, New Castle, United Kingdom) antibodies, subsequently captured on goat antimouse IgG-coated paramagnetic beads (Dynal Biotech, Oslo, Norway), and used directly for RNA isolation.
EG7 thymoma cells transfected with OVA where cultured in RPMI (Invitrogen) supplemented with 10% FCS (Perbio Science), 25 mM HEPES buffer, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM sodium pyruvate, 1 times nonessential amino acids, and 50 μM 2-mercaptoethanol (all from Invitrogen). Cells were maintained with addition of 40 μg/mL G418. Before injection in mice, G418 concentration was increased to 400 μg/mL for 2 rounds of expansion.
Recombinant mouse IL-21 protein was obtained as previously described.40 Recombinant mouse IL-2 was obtained from R&D Systems (Minneapolis, MN). Anginex was kindly provided by Dr K. Mayo (Department of Biochemistry, University of Minnesota, Minneapolis, MN).
In vitro and ex vivo sprouting assay
In vitro sprouting and tube formation were studied using Cytodex-3 beads overgrown with SVEC4–10 ECs in a three-dimensional collagen gel (PureCol; INAMED Biomaterials, Fremont, CA) containing 10 ng/mL bFGF as described previously.41 Sprout formation was stimulated with medium containing 10 ng/mL bFGF and 40 ng/mL VEGF. Recombinant IL-21, IL-2, or anginex was added in the medium on top of the gel. After 72 hours the length of the sprouts was analyzed.
Ex vivo sprouting was investigated by using rings of the mouse thoracic aorta as was described earlier34 ; 1-mm-thick cross-sectional rings were placed in the wells of a 96-well tissue culture plate in the previously described collagen gel. Medium (DMEM, 20% FCS, 2 mM l-glutamine, 2 mM sodium pyruvate, 20 mM HEPES, 1% nonessential amino acids, 1.5% MEM vitamins, antibiotics, 1 IU/100 mL heparin [Invitrogen]) containing recombinant IL-21, IL-2, or anginex was put on top of the gels. Vascular sprouting from each ring was examined using an inverted microscope. The width of the tube formation area was measured at 3 different predefined places of the aortic ring. Different culture conditions were tested at least in triplicate.
Flow cytometry
ECs were fixed with 1% paraformaldehyde for 30 minutes at room temperature. IL-21 receptor-α (IL-21-Rα) expression was detected biotinylated mAb to IL-21R-α.10 Subsequently, the cell suspensions were incubated with phycoerythrin-labeled streptavidin (Dako North America). Stained cells were analyzed on a FACSscan flow cytometer using Cell Quest software (BD Biosciences).
Proliferation and apoptosis measurement
EC proliferation was measured using a [3H]thymidine (GE Healthcare, Little Chalfont, United Kingdom) incorporation assay as described previously.41 Angiogenically stimulated SVEC4-10 were exposed for 3 days to recombinant mouse IL-21, IL-2, or anginex before proliferation was assessed. At least 3 independent experiments were performed. In each experiment, measurements were done in triplicate.
Apoptosis was measured by propidium iodide (Brunschwig Chemie, Amsterdam, The Netherlands) staining as described previously.41 bFGF (10 ng/mL) stimulated cells were cultured for 3 days in the presence or absence of IL-21, IL-2, or anginex. After this period, the cells were harvested, fixed in 70% ethanol, centrifuged, resuspended in DNA extraction buffer, and incubated for 20 minutes at 37°C. After incubation, propidium iodide was added at a final concentration of 20 μg/mL, and the DNA profile was directly analyzed with the FACScalibur.
Migration measurement
Migration of murine ECs was measured using the wound assay.34 In brief, SVEC4-10 cells were seeded in gelatin-coated cell culture plates (Corning Life Sciences) and grown to confluence. Using a blunt pipette tip, a cross-shaped wound was made in the well. Cells were washed with phosphate-buffered saline (PBS) and fresh medium containing 10 ng/mL bFGF with or without IL-21, IL-2, or anginex. Wound width was measured at 4 predefined locations at start and at 2, 4, 6, 8, and 24 hours after wounding.
Chorioallantoic membrane assay
The chorioallantoic membrane (CAM) assay was performed in fertilized White Leghorn eggs as described previously.41 In brief, CAMs were treated by daily addition of sterile PBS, recombinant mouse IL-21, or IL-2 from days 10 to 13. On day 14, the CAMs were photographed. Quantification of vascularization was done by enumeration of intersections with 5 concentric rings that were superimposed on the photographs.
Mouse tumor model
C57Bl/6 mice were injected subcutaneously at the base of the spine with 5 × 106 mouse EG7 tumor cells on days 0. On days 10, 12, 14, 16, and 18 after tumor inoculation, mice were treated with intraperitoneal injections of either PBS, 2000 IU IL-2 or 20 μg IL-21 in a volume of 500 μL. At day 20, tumors were excised and snap frozen in liquid nitrogen.
Immunohistochemistry
Immunohistochemical staining was performed on frozen sections (5 μm), fixed in acetone on ice, and air dried. Immersing the slides in 0.3% hydrogen peroxidase in PBS for 30 minutes blocked endogenous peroxidase. After washing 3 times with PBS, aspecific binding was blocked by PBS containing 20% FCS and 0.1% Tween 20 for 15 minutes. The sections were incubated with rat anti mouse CD31 (BD Biosciences PharMingen, San Diego, CA) primary antibody for 1 hour, followed by incubation with peroxidase-labeled goat anti–rat IgG antibody (Immunotech, Marseille, France). The peroxidase activity was detected using diaminobenzidine (Sigma-Aldrich).
Quantitative real time RT-PCR
Total RNA isolation from cultured cells, cDNA synthesis, and quantitative real-time RT-PCR (qRT-PCR) were performed essentially as described previously42 using iQ SYBR Green PCR Supermix (Bio-Rad, Hercules, CA). The expression of each target gene was normalized to the expression of the control gene cyclophilin A by calculation of dCt values (Ct target − Ct reference gene). Primer sequences are shown in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Western blot analysis
A total of 10 ng/mL of bFGF stimulated SVEC4-10 cells or primary mouse ECs were incubated with IL-2, IL-21, or anginex for 5 or 30 minutes. Cells were harvested and permeabilized in NP40 lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% NP40, 1 mM Na3VO4, 5 mM NaF, 1 mM 2-aminoethyl-benzenesulfonylfluoride, 0.8 mM aprotinin, 21 mM leupeptin, 36 mM bestatin, 15 mM pepstatin A, and 14 mM E-64). Whole cell lysates (∼10 μg/sample) were fractionated on 4% to 12% polyacrylamide gels (Invitrogen) and Western blotted. Blots were probed with antibodies to phosphorylated Stat1 (Y701), Stat3 (Y705), Stat5 (Y694 for Stat5a and Y699 for Stat5b; Cell Signaling Technology, Danvers, MA), and then reprobed with antibodies to Stat1, Stat3, Stat5a, Stat5b (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analysis
All data are expressed with SEM and were statistically analyzed using Mann-Whitney U tests (using SPSS-10 software). Probabilities less than .05 were considered statistically significant.
The Institutional Review Board of Maastricht University and University Hospital, Maastricht, approved this study.
Results
IL-21 inhibits sprout formation by ECs
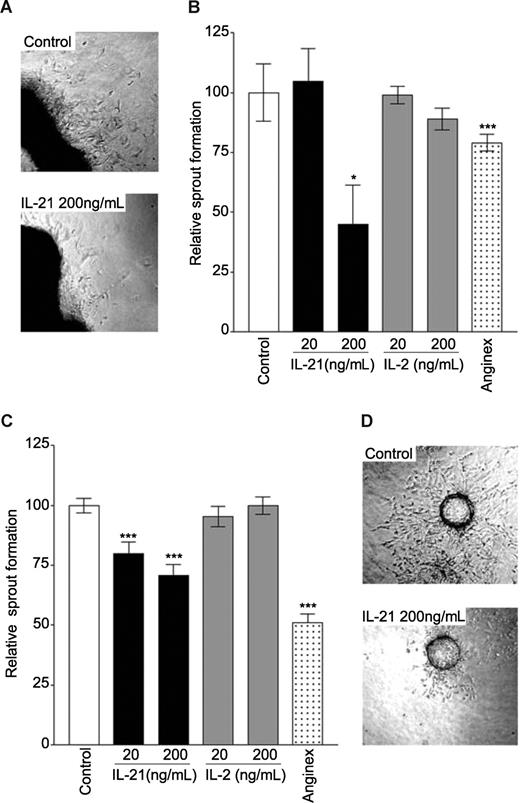
The sprouting of primary aortic ECs into a three-dimensional seminatural collagen matrix was inhibited by approximately 60% in the presence of IL-21 (200 ng/mL, Figure 1A,B). In the present study, we compared the effects of IL-21 with those of IL-2, a member of the same cytokine family and previously suggested to inhibit blood vessel formation. Interestingly, treatment with recombinant mouse IL-2 in this assay did not lead to significantly inhibited sprout formation by mouse aortic ECs (Figure 1B).
IL-21 inhibits angiogenic sprouting of endothelial cells. (A) A mouse aortic ring treated with bFGF (10 ng/mL) and bFGF and IL-21 200 ng/mL. (B) Quantification of sprouting of primary mouse endothelial cells from isolated aortic rings in a three-dimensional collagen matrix. (C) Sprouting of bFGF (10 ng/mL) stimulated SVEC4-10 cells (mouse endothelial cell line) grown on beads embedded in a three-dimensional collagen matrix. (D) An example of sprouting of SVEC4-10 cells treated with bFGF (10 ng/mL, top panel) and bFGF and IL-21 (bottom panel). Data are relative mean values of at least 3 experiments (***P < .0005, *P < .05, compared with control).
IL-21 inhibits angiogenic sprouting of endothelial cells. (A) A mouse aortic ring treated with bFGF (10 ng/mL) and bFGF and IL-21 200 ng/mL. (B) Quantification of sprouting of primary mouse endothelial cells from isolated aortic rings in a three-dimensional collagen matrix. (C) Sprouting of bFGF (10 ng/mL) stimulated SVEC4-10 cells (mouse endothelial cell line) grown on beads embedded in a three-dimensional collagen matrix. (D) An example of sprouting of SVEC4-10 cells treated with bFGF (10 ng/mL, top panel) and bFGF and IL-21 (bottom panel). Data are relative mean values of at least 3 experiments (***P < .0005, *P < .05, compared with control).
A similar in vitro assay using the SVEC4-10 cell line was used to demonstrate a direct effect on EC. This murine cell line is an excellent tool for angiogenesis research because it is inducible by angiogenic growth factors.34 Addition of IL-21 inhibited bFGF-induced sprouting by 20% to 30% (Figure 1C,D). Similar as in the aortic ring assay, IL-2 did not inhibit the sprouting of SVEC cells. The positive control anginex reduced the sprout formation by approximately 50% (Figure 1C).
The IL-21 receptor is expressed by EC
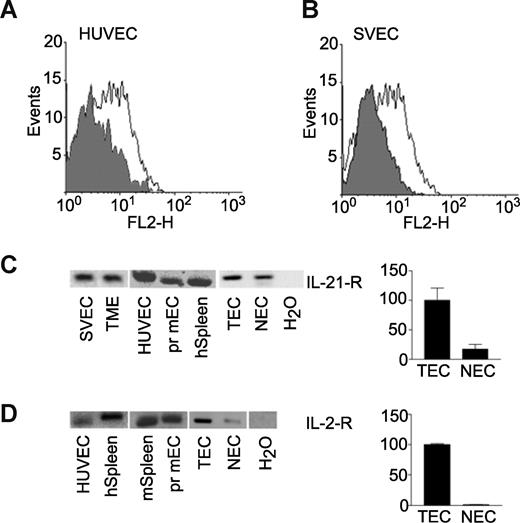
Flow cytometry identified the IL-21 receptor to be expressed by SVEC. Similar results were found for primary human ECs (HUVEC, Figure 2A,B). These data were confirmed for various EC lines at the transcriptional level by qRT-PCR. The expression level of the IL-21 receptor was low in the ECs compared with the positive control spleen (average dCt values of 17 for EC samples vs 10 for spleen) but within the boundaries of our assay. Analysis of the PCR products on agarose gel showed unambiguously the presence of the IL-21 receptor α-chain in primary human and mouse ECs and EC lines (Figure 2C). In addition, we tested the mRNA expression of the IL-21 receptor on freshly isolated human tumor ECs and normal cells.39 Although expression was low (average dCt = 11), a clear expression of IL-21 receptor could be detected when we analyzed the qRT-PCR on agarose gel (Figure 2C).
IL-21 receptor α-chain is expressed by endothelial cells. (A) FACS analysis of human umbilical vein-derived endothelial cells (HUVECs) stained for IL-21-R α-chain (white), conjugate control is represented by gray histogram. (B) SVEC4–10 stained for IL-21-Rα. (C) mRNA expression of IL-21-Rα mouse endothelial cell lines SVEC and TME in primary (pr) human (h) and mouse (m) endothelial cells, and freshly isolated human tumor (TEC) and normal endothelial cells (NECs).39 (D) mRNA expression of IL-2-Rα in primary human, primary mouse, and freshly isolated human tumor and normal endothelial cells. Positive control is spleen tissue; negative control is no template in the PCR reaction (H2O).
IL-21 receptor α-chain is expressed by endothelial cells. (A) FACS analysis of human umbilical vein-derived endothelial cells (HUVECs) stained for IL-21-R α-chain (white), conjugate control is represented by gray histogram. (B) SVEC4–10 stained for IL-21-Rα. (C) mRNA expression of IL-21-Rα mouse endothelial cell lines SVEC and TME in primary (pr) human (h) and mouse (m) endothelial cells, and freshly isolated human tumor (TEC) and normal endothelial cells (NECs).39 (D) mRNA expression of IL-2-Rα in primary human, primary mouse, and freshly isolated human tumor and normal endothelial cells. Positive control is spleen tissue; negative control is no template in the PCR reaction (H2O).
The expression of the IL-21 receptor α-chain on ECs suggests that the inhibitory effects by IL-21 on sprout formation as described in the previous section occur via a direct effect on the ECs. Similarly, the presence of the IL-21 receptor on human tumor ECs suggests that IL-21 has a direct effect on ECs in tumors.
Analysis of the IL-2 receptor α-chain by qRT-PCR demonstrated that this receptor is also present on various types of ECs (Figure 2D). These data together demonstrate that the tested ECs can directly respond to IL-21 and IL-2 treatment via their specific receptor on the cell membrane.
IL-21 and IL-2 inhibit growth factor-induced proliferation but not migration of ECs
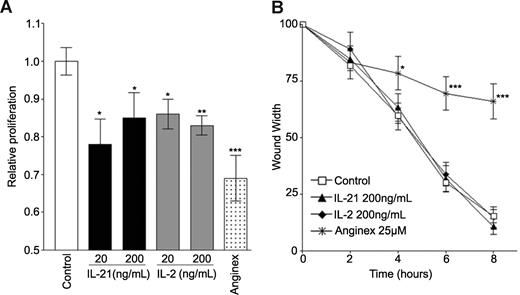
To further investigate the mechanism by which IL-21 caused inhibition of EC sprouting and thus angiogenesis in vitro, the antiproliferative activity of IL-21 was tested in a 3H-thymidine incorporation assay using SVEC4-10 cells. Cells were stimulated with bFGF and cultured for 3 days in the presence of IL-21, IL-2, or anginex. A slight but significant reduction of EC proliferation by approximately 20% was observed after treatment with IL-21. Comparable results were found for IL-2 (Figure 3A). The inhibition of proliferation by IL-21 and IL-2 was not associated with induction of cell death, as flow cytometric analysis of DNA fragmentation showed, in contrast to the positive control anginex,34 no increase of apoptotic or necrotic cells after IL-21 or IL-2 treatment (data not shown).
Proliferation but not migration is inhibited by IL-21 or IL-2 treatment. (A) Proliferation of angiogenically stimulated (bFGF 10 ng/mL) SVEC4-10 is inhibited by 3 days of treatment with IL-21, IL-2, or anginex. (B) Wound assay of SVEC4-10 cells. Migration of EC is not effected by treatment with IL-21 or IL-2. The angiogenesis inhibitor anginex inhibits migration of SVEC4-10 by approximately 70%. Data are relative mean values of at least 4 experiments (***P < .0005, **P < .005, *P < .05, compared with control).
Proliferation but not migration is inhibited by IL-21 or IL-2 treatment. (A) Proliferation of angiogenically stimulated (bFGF 10 ng/mL) SVEC4-10 is inhibited by 3 days of treatment with IL-21, IL-2, or anginex. (B) Wound assay of SVEC4-10 cells. Migration of EC is not effected by treatment with IL-21 or IL-2. The angiogenesis inhibitor anginex inhibits migration of SVEC4-10 by approximately 70%. Data are relative mean values of at least 4 experiments (***P < .0005, **P < .005, *P < .05, compared with control).
Next to proliferation, migration of ECs is a key process in the formation of new blood vessels during angiogenesis. EC migration was investigated using the wound assay. Whereas anginex inhibited the wound closure efficiently, IL-21 as well as IL-2 did not have an effect. It is concluded that IL-21 does not affect migration of EC (Figure 3B).
IL-21 and IL-2 affect in vivo angiogenesis in the chick embryo CAM assay
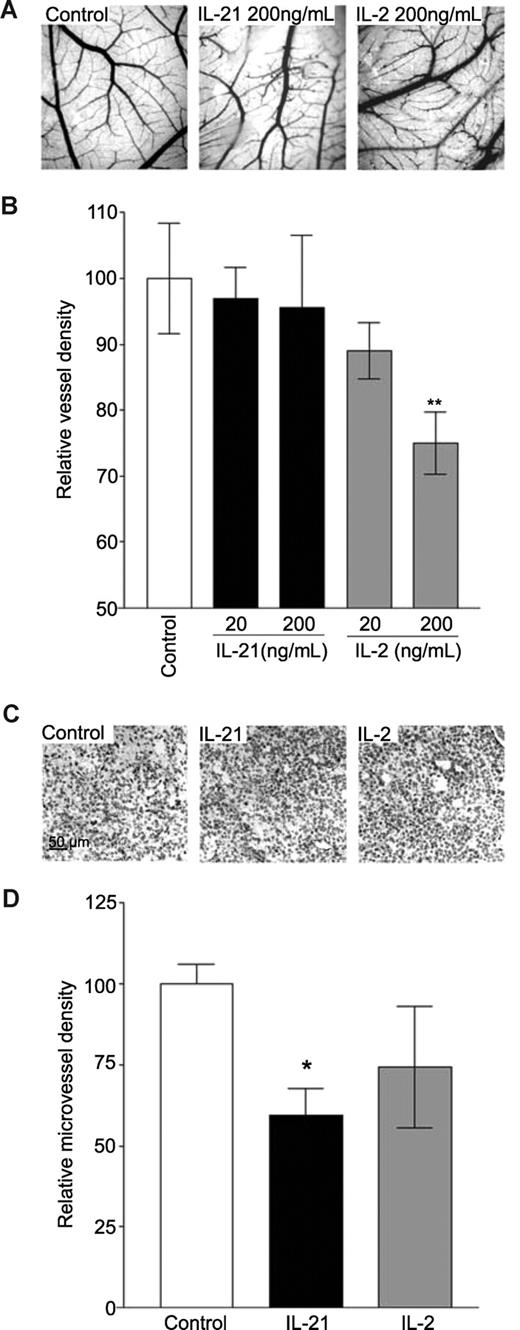
In vivo angiogenesis was first investigated in the CAM of the chicken embryo, an assay that represents developmental angiogenesis. Although treatment of the CAMs with IL-21 did not reduce vessel density (Figure 4B), a disturbed, tortuous and irregular growth of vessels was detected compared with control CAMs (Figure 4A). Recombinant IL-2 showed a similarly disturbed vessel outgrowth. In addition, IL-2 did reduce vessel density in the CAMs by 25% (Figure 4A,B). This response to IL-2 confirms results presented in earlier reports.36
IL-21 and IL-2 inhibit angiogenesis in vivo. (A,B) Treatment of CAMs with 200 ng/mL IL-2 reduces vessel formation in CAMs, whereas IL-21 does not affect the number of vessels in CAMs (n = 7 for each group). Morphologic examination shows that vessels in CAMs treated with 20 ng/mL or 200 ng/mL IL-21 are tortuous and irregular compared with control CAMs (B). (C) Images of CD31 staining of EF7 tumor sections of mice treated with PBS (control), 20 μg of IL-21, or 2000 IU of IL-2 (images seen through a Leica DM5000B microscope, Leica Microsystems, Rijswijk, The Netherlands). (D) IL-2 (n = 4) and IL-21 (n = 5) treatment reduced microvessel density in tumor tissues compared with control (PBS, n = 5). Results are relative mean values (**P < .005, *P < .05, compared with control).
IL-21 and IL-2 inhibit angiogenesis in vivo. (A,B) Treatment of CAMs with 200 ng/mL IL-2 reduces vessel formation in CAMs, whereas IL-21 does not affect the number of vessels in CAMs (n = 7 for each group). Morphologic examination shows that vessels in CAMs treated with 20 ng/mL or 200 ng/mL IL-21 are tortuous and irregular compared with control CAMs (B). (C) Images of CD31 staining of EF7 tumor sections of mice treated with PBS (control), 20 μg of IL-21, or 2000 IU of IL-2 (images seen through a Leica DM5000B microscope, Leica Microsystems, Rijswijk, The Netherlands). (D) IL-2 (n = 4) and IL-21 (n = 5) treatment reduced microvessel density in tumor tissues compared with control (PBS, n = 5). Results are relative mean values (**P < .005, *P < .05, compared with control).
IL-21 inhibits tumor angiogenesis in vivo
Effects of IL-21 and IL-2 on tumor angiogenesis in vivo were determined by treatment of EG7 tumor-bearing mice with IL-21, IL-2, or PBS (control) at concentrations that have previously been shown to be effective (Figure 4C).40 Immunohistochemical staining of tumor sections of mice treated with recombinant mouse IL-21 using CD31 antibody showed a significantly decreased microvessel density, compared with PBS-treated mice (Figure 4C,D). Treatment of mice with IL-2 showed a decreased vessel formation in tumors as well, but this inhibition was not found to be statistically significant (Figure 4C,D).
IL-21 and IL-2 regulate expression of angiogenesis-related genes in activated EC
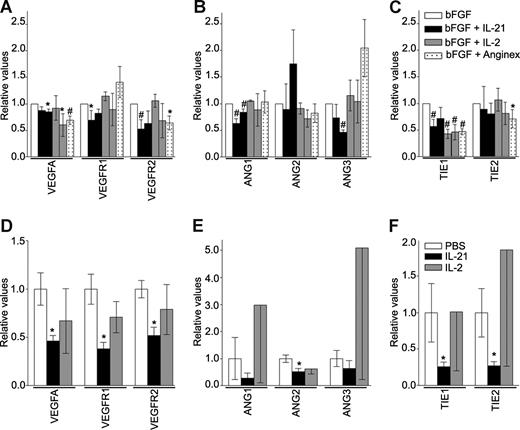
To investigate the feature of the angiostatic response of EC after exposure to IL-21 and IL-2, we performed qRT-PCR on samples of in vitro cultured ECs and the aforementioned mouse tumor tissues. Absolute Ct values can be found in Table S2. In cultured ECs, the major angiogenesis-related growth factor vascular endothelial growth factor A (VEGFA) and both of its receptors VEGFR1 and VEGFR2 are significantly decreased by exposure to IL-21 compared with bFGF treatment alone (Figure 5A). The IL-21–induced down-regulation of VEGF-A and VEGFR2 is shared with the angiogenesis inhibitor anginex (Figure 5A). The expression of angiopoietins, mediators of vessel sprouting, maturation, and remodeling, is also affected by IL-21 treatment in vitro. Angiopoietin-1and -3 (ANG1, ANG3) show a lower expression after IL-21 treatment (Figure 5B), whereas neither IL-2 nor anginex treatment shows this effect in ECs. The expression of the tyrosine kinase receptor-1 (TIE1), one of both receptors of the angiopoietin family, is diminished after IL-21 treatment, an effect that is shared with IL-2 and anginex in ECs (Figure 5C). These results support the observation of antiangiogenesis activity by IL-21 and IL-2. In tumor tissues of mice treated with IL-21, we also observed a significant decrease of VEGFA and both receptors VEGFR1 and VEGFR2 compared with PBS treatment (Figure 5D). The mRNA expression of angiopoietins is also reduced on IL-21 treatment (Figure 5E), although only significantly for angiopoietin-2. A significant decline of approximately 75% is determined for TIE1 and TIE2 expression in tumor tissues of IL-21–treated mice (Figure 5F). In experiments, treatment with IL-2 did never induce significant changes of expression.
IL-21 and IL-2 regulate expression of angiogenesis-related genes in activated ECs and tumor tissues. (A-C) Regulation of mRNA expression levels of angiogenesis factors in bFGF (10 ng/mL) stimulated SVEC4-10 after 3 days of IL-21, IL-2, or anginex treatment. Concentrations of the compounds are as indicated in previous figures: first bar represents 20 ng/mL; second bar, 200 ng/mL and anginex 25 μM. Results are relative means of at least 3 experiments (*P < .05, #P < .005, compared with bFGF). (D-F) Regulation of mRNA expression levels of angiogenesis factors in tumor tissues of EG7 tumors after PBS (n = 4), IL-21 (20 μg, n = 4), or IL-2 (IU 2000, n = 3) treatment. Results are relative means (*P < .05).
IL-21 and IL-2 regulate expression of angiogenesis-related genes in activated ECs and tumor tissues. (A-C) Regulation of mRNA expression levels of angiogenesis factors in bFGF (10 ng/mL) stimulated SVEC4-10 after 3 days of IL-21, IL-2, or anginex treatment. Concentrations of the compounds are as indicated in previous figures: first bar represents 20 ng/mL; second bar, 200 ng/mL and anginex 25 μM. Results are relative means of at least 3 experiments (*P < .05, #P < .005, compared with bFGF). (D-F) Regulation of mRNA expression levels of angiogenesis factors in tumor tissues of EG7 tumors after PBS (n = 4), IL-21 (20 μg, n = 4), or IL-2 (IU 2000, n = 3) treatment. Results are relative means (*P < .05).
Regulation of Stat phosphorylation in EC after IL-21 treatment
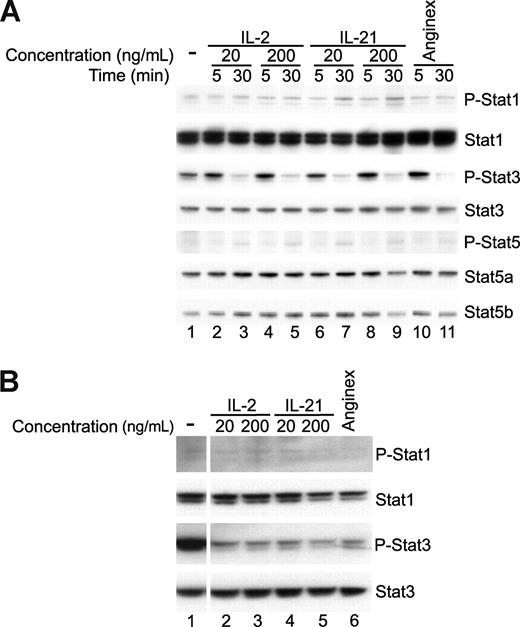
In leukocytes, IL-21 signals through the IL-21-R and the JAK/Stat pathway. JAK1 and/or JAK3 can activate Stat1, Stat3, and to a lesser extent Stat5 by phosphorylation after induction by IL-21. To investigate whether similar signaling can occur in ECs, we performed Western blot analysis on protein lysates of SVEC4-10 (Figure 6A) and primary mouse ECs (Figure 6B).
Decreased Stat3 activation is correlated with angiostatic activity. (A) Western blot analysis (n = 2) of cell lysates of 10 ng/mL bFGF stimulated SVEC4-10 after 5- or 30-minute incubation with IL-2, IL-21, or anginex. After 30 minutes, IL-2-, IL-21-, and anginex-treated cells showed decreased Stat3 phosphorylation (P-Stat3). (B) Western blot analysis (n = 1) of cell lysates of bFGF stimulated primary mouse endothelial cells after 30 minutes with IL-21, IL-2, or anginex.
Decreased Stat3 activation is correlated with angiostatic activity. (A) Western blot analysis (n = 2) of cell lysates of 10 ng/mL bFGF stimulated SVEC4-10 after 5- or 30-minute incubation with IL-2, IL-21, or anginex. After 30 minutes, IL-2-, IL-21-, and anginex-treated cells showed decreased Stat3 phosphorylation (P-Stat3). (B) Western blot analysis (n = 1) of cell lysates of bFGF stimulated primary mouse endothelial cells after 30 minutes with IL-21, IL-2, or anginex.
Western blot analysis of bFGF-stimulated SVEC4-10 showed presence of activated (phosphorylated) Stat3 (P-Stat3) and a slight activation of Stat1 (P-Stat1, Figure 6A lane 1). Interestingly, incubation of SVEC4-10 for 30 minutes with IL-21 induced a dramatic and significant decrease in the expression of phosphorylated Stat3, a feature shared with the angiogenesis inhibitor anginex. Similar effects were observed for IL-2. This regulation was not visible after 5 minutes of exposure to IL-21, IL-2, or anginex.
Primary mouse ECs showed expression of P-Stat3 after bFGF stimulation as well (Figure 6B lane 1). In addition, in these primary ECs, phosphorylation of Stat3 was diminished after 30 minutes of exposure to the compounds (Figure 6B lanes 2-6). No differences could be detected for the other Stat proteins tested. Because Stat3 activation has been implicated in angiogenesis before,21,22 the diminishing effects of IL-21 and IL-2 on Stat3 phosphorylation reinforce the angiostatic activities of both IL-21 and IL-2. In addition, they implicate the role of diminished STAT3 activation in the observed antiangiogenesis effects.
Discussion
IL-21 is a pleiotropic cytokine with immune-regulatory properties and immune-therapeutic capacity. In this study, we have identified a novel function for IL-21. We demonstrate that the IL-21 receptor is present on normal and tumor ECs and can mediate angiostatic properties by several in vitro assays. Although IL-21 moderately inhibits the proliferation of ECs, it markedly inhibited sprouting of angiogenically stimulated ECs. In addition, it also reduces vessel formation and disturbs vessel architecture in vivo. IL-21 was found to reduce the expression of VEGFR2 and TIE1 in ECs, contributing to the lower angiogenic profile. In addition, exposure of ECs to IL-21 led to a decrease in expression of VEGF-A, ANG1, and ANG3. Similar results on gene expression were found when testing samples of intact tumor tissue, indicating that the effects are shared by other cells, including the tumor cells as well. The results of the current report are in line with an earlier report on disruption of the vascular network as found in tumors of mice treated with IL-21.43 Furthermore, the decrease of Stat3 phosphorylation in activated ECs after treatment with IL-21, an effect that is shared with the angiogenesis inhibitor anginex, suggests that angiostatic signaling by IL-21 in EC is mediated through Stat3.
The current report also confirms the earlier described angiostatic power of IL-2. This type I cytokine reduced proliferation of angiogenically stimulated ECs in vitro as well as vessel formation in vivo. In contrast to IL-21, IL-2 did not reduce sprouting of aortic rings or of ECs. This difference, and the fact that IL-21 seems to be a more powerful angiogenesis inhibitor than IL-2, could be explained by differential expression of ANG1, ANG3, and TIE1 in ECs induced by IL-21 and IL-2 treatment. Angiopoietins and their receptors are essential for vessel sprouting, remodeling, and maturation.44 A decrease of their expression might interfere with these processes during angiogenesis. This is also in line with our observations in the CAM assay that showed a disturbed and tortuous character of the vessels after IL-21 treatment. Interestingly, IL-21 also decreases the expression of VEGFR1 in ECs. This is also consistent with the observed inhibition of sprouting and disturbed vessel morphology because a defect in VEGFR1 can lead to the assembly of ECs into abnormal vascular channels.45 However, we could detect irregular vessel morphology in the CAMs after IL-2 treatment as well, indicating that other factors, eg, nitric oxide,36 might be involved in the disturbed vessel outgrowth in CAMs as well. These data suggest that IL-2 is probably involved in survival rather than in differentiation mechanisms, which IL-21 seems to be. The regulation of VEGFA, VEGFR1, and VEGFR2 gene expression in tumor tissues is similar for both IL-21 and IL-2, although the effects of IL-21 are stronger. In addition to these VEGF related genes, IL-21 decreases the expression of the angiopoietin family as well. This differential regulation of gene expression can explain the predominant effects of IL-21 over IL-2, on tumor angiogenesis. Because IL-2 and IL-21 both affect hematopoietic cells differently,2,40 these cells, when present, for example, in the ex vivo sprouting assay and the tumor growth experiment, could mediate the effects of the cytokines on the endothelium. The clear effects in the cultured EC experiments favors the view of different effects directly on the endothelium.
Here we show that γc-dependent cytokines can have different effects on endothelium. For IL-15, it has been shown that it can induce angiogenesis.46,47 Our results demonstrate that both IL-2 and IL-21 can inhibit angiogenesis, but in a different way.
As mentioned in the Introduction, IL-21 can have different effects on leukocytes and thereby affect their proangiogenic function. This can have implications on tumor angiogenesis because IL-21 might reduce the proangiogenic factors secreted by leukocytes. However, the observed expression of the IL-21 receptor on tumor ECs supports the idea that microvessel density is decreased by a direct negative effect of IL-21 on tumor ECs.
In leukocytes, IL-21 signaling has been shown to occur via the Janus family tyrosine kinases, JAK1 and JAK3. These kinases then mediate activation of Stat1, Stat3, and to a lesser extent Stat5a and Stat5b.2 The activation of a specific Stat molecule by IL-21 depends on the leukocyte subgroup.2,19 We reasoned that similar signaling may occur in ECs and therefore tested whether phosphorylated Stat3 is detectable in ECs. To our surprise, phosphorylated Stat3 is present in activated ECs. In addition, Stat3 phosphorylation is decreased in ECs after 30 minutes of incubation with either IL-21 or IL-2, an effect that is shared with the angiostatic compound anginex. Interestingly, phosphorylated Stat3 in EC has recently been linked to the induction of angiogenesis.21,22 Thus, angiogenesis inhibitors, including IL-21, IL-2, and anginex, may exert their effect, at least in part, by reducing Stat3 phosphorylation. Inactivation of Stat3 in tumor cells has already been implied to be a promising strategy for therapy in various cancer types by inhibiting tumor-derived VEGF action and angiogenesis.48-50 Our data suggest that inhibition of Stat3 could also inhibit tumor angiogenesis by a direct effect on ECs.
In conclusion, the ability of IL-21 to inhibit angiogenesis in vitro and in vivo, in combination with its previously reported beneficial effect on survival of tumor-bearing mice,40 provides further impetus for the use of IL-21 in therapeutic approaches for cancer. In addition, further elucidation of the role of Stat3 phosphorylation in tumor angiogenesis may provide additional clues for the development of novel and more potent angiogenesis inhibitors and their mechanism of action.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.C. designed research, performed experiments, collected data, analyzed and interpreted data, performed statistical analysis, and wrote the paper; S.P.T. designed research, performed experiments, interpreted data, and wrote the paper; R.Z. and C.E. performed experiments; J.R.v.B. performed experiments and wrote the paper; W.J.L. and P.A.S. wrote the paper; and A.W.G. designed research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arjan W. Griffioen, Angiogenesis Laboratory, Department of Pathology, University Hospital Maastricht, PO Box 5800, 6202AZ Maastricht, The Netherlands; e-mail: aw.griffioen@path.unimaas.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal