Abstract
A major limitation of current lentiviral vectors (LVs) is their inability to govern efficient gene transfer into quiescent cells such as primary T cells, which hampers their application for gene therapy. Here we generated high-titer LVs incorporating Edmonston measles virus (MV) glycoproteins H and F on their surface. They allowed efficient transduction through the MV receptors, SLAM and CD46, both present on blood T cells. Indeed, these H/F-displaying vectors outperformed by far VSV-G-LVs for the transduction of IL-7–prestimulated T cells. More importantly, a single exposure to these H/F-LVs allowed efficient gene transfer in quiescent T cells, which are not permissive for VSV-G-LVs that need cell-cycle entry into the G1b phase for efficient transduction. High-level transduction of resting memory (50%) and naive (11%) T cells with H/F-LVs, which seemed to occur mainly through SLAM, was not at cost of cell-cycle entry or of target T-cell activation. Finally, the naive or memory phenotypes of transduced resting T cells were maintained and no changes in cytokine profiles were detected, suggesting that T-cell populations were not skewed. Thus, H/F-LV transduction of resting T cells overcomes the limitation of current lentiviral vectors and may improve the efficacy of T cell–based gene therapy.
Introduction
Efficient gene transfer into T lymphocytes may allow the treatment of several genetic dysfunctions of the hematopoietic system, such as severe combined immunodeficiency, and the development of novel therapeutic strategies for diseases such as cancers and acquired diseases such as AIDS.1-5 Lentiviral vectors (LVs) can transduce many types of nonproliferating cells,6,7 with the exception of some particular quiescent cell types such as hematopoietic stem cells in G0 phase of the cell cycle,8 monocytes,9 and resting T cells.10 Completion of reverse transcription, nuclear import, and subsequent integration of the lentivirus genome do not occur in these cells11,12 unless they are activated via the T-cell receptor (TCR) or by cytokines inducing resting T cells to enter in G1b phase of the cell cycle.11,13-15 Importantly, TCR stimulation leads to severe skewing of T cells.15,16 It is, however, of utmost importance that the responses of T cells to antigens are not dramatically altered by the gene transfer protocol. Naive T cells provide a long-lasting immune reconstitution to patients, so these are the cells that need to be transduced for effective gene therapy without affecting their phenotype. Now it is clear that use of the survival cytokines, IL-2 or IL-7, allows an efficient lentiviral vector gene transfer and could preserve a functional T-cell repertoire without skewing the T-cell populations.13,15,17,18 IL-7 is an appealing candidate because it functions as a master candidate of T-cell survival and homeostatic proliferation.19,20 Indeed, IL-7 treatment induces cord blood naive T cells to proliferate, whereas neither naive nor memory adult T cells proliferate upon IL-7 treatment. IL-7–stimulated memory T cells progress into G1b phase of the cell cycle, whereas naive T cells enter only into G1a phase and thus are not permissive to VSV-G-LVs. Long-term IL-7 stimulation, however, induces also naive T cells to partially enter into G1b phase and renders them permissive.10,15
The measles virus (MV) belongs to the paramyxoviruses causing measles disease. It propagates mainly via a cell-to-cell fusion mechanism, using its 2 surface glycoproteins: hemagglutinin (H) that binds to the cellular receptor of the host cell and fusion protein (F) that mediates fusion of the viral and the cellular membranes.21 The natural receptor of MV, used by most clinical isolates, is the signaling lymphocyte activating molecule (SLAM).22-24 SLAM is constitutively expressed in ROhigh memory T cells, immature thymocytes, a proportion of B cells, macrophages, and dendritic cells.25,26 Vaccine strains also gained host cell entry through the hCD46 receptor,27 a member of the family of the complement activation regulators, expressed on all human nucleated cells.28
The F protein is a type I membrane protein with an N-terminal ectodomain that has to be cleaved into the F1 and F2 subunits to allow pH-independent fusion.29 Cleaved F trimers need to interact with H glycoproteins (gps) to give a biologically active MV glycoprotein complex. Membrane-proximal regions in the ectodomains of both proteins are assumed to be involved in the formation of fusogenic H-F complexes. The cytoplasmic tails (CTs) of both H and F are clearly involved in virus assembly, since they bind to the matrix (M) protein that acts as a bridge between the viral envelope and nucleocapsid.30 However, it is possible to recover recombinant MVs with shortened CTs.31,32
It is now clear that LVs can be pseudotyped with heterologous envelope gps, giving rise to viral particles with altered tropism that still keep their ability to transduce nondividing cells. LVs have been pseudotyped with many different viral envelope proteins or chimeric gps including VSV-G, MLV, RD114, or HTLV-1.15,33-35
Here we displayed efficiently the MV glycoproteins, H and F, at the surface of lentiviral vectors resulting in high-titer vectors that conserved the MV tropism. These novel H/F lentiviral vector pseudotypes outperformed by far VSV-G-LVs even at low vector doses for the transduction of rIL-7–stimulated T cells, without changing their phenotype. Most importantly, the novel H/F-pseudotyped LVs were able to transduce completely quiescent adult T cells in the absence of any exogenous stimulus, where VSV-G pseudotypes remained refractory. The efficient transduction levels of resting T cells (50%) were not at cost of cell-cycle entry and maintained the T-cell memory and naive phenotypes.
Methods
Plasmids and constructs
All wt and mutant viral glycoproteins H and F (Edmonston MV strain) are inserted into pCG plasmid under the control of the cytomegalovirus early promoter. The plasmids pCG-HΔ15, pCG-HΔ20, pCG-HΔ24 encoding for cytoplasmic tail mutants of H, and pCG-FΔ30 were a kind gift of A. Maisner. pCG-H97E encodes a mutant MV H protein (K97E) that does not induce syncytia formation with F (S.J.R., L. Hallak, and C. Hu, unpublished data, February 2007). pCMV-G and RDTR plasmids are described elsewhere.35,36
Antibodies
Conformational-sensitive antibodies used for the binding assays Y503 (anti-F) and Y55 (anti-H) were a kind gift from D. Gerlier. The CD46-blocking antibody mAb13/42 was a kind gift from R. Buckland. The SLAM-blocking antibody clone A12 was purchased from eBioscience (Cliniscience, Montrouge, France), whereas anti–CD46-PE and anti–SLAM-PE antibodies were purchased from BD Biosciences (Le Pont de Claix, France). T-cell phenotyping was performed with anti–CD45RA-APC, anti–CD45RO-PE, anti–CD62L-PECy7, anti–CCR7-PE, anti–CD25-PE, anti–CD69-PE, anti–HLA-DR-PE, and anti–CD71-PE purchased from BD Biosciences, whereas anti–CD4-APC was obtained from Miltenyi Biotech (Bergisch Gladbach, Germany) and anti–CD8-PERCP-PECY5.5, from eBioscience (Cliniscience).
Production of retroviral vectors and titering
MLV-based vectors and self-inactivating HIV-1–derived vectors were generated by transient transfection of 293T cells as described previously.37 Slight modifications were introduced: 3 μg pCMV-G plasmid or 7 μg pCMV-RDTR plasmid was transfected; for codisplay of the different H and F gps, 3 μg of each envelope plasmid was transfected. Viral supernatant was harvested 24 or 48 hours after transfection. Low-speed concentration of the vectors was performed by overnight centrifugation of the viral supernatant at 3000g at 4°C. To determine transduction efficiency and infectious titers of the MLV and HIV vectors, serial dilutions of vector preparations were added to 293T cells. The infectious titers are expressed as 293T transducing units per milliliter (TU/mL).
Cell lines and primary cells
Jurkat (T-cell line), Raji (B-cell line), and B95a (marmoset B-cell line transformed with Epstein-Barr virus) were grown in RPMI medium (GibcoBRL Invitrogen, Auckland, New Zealand) supplemented with 10% FCS and 50 μg/mL penicillin/streptomycin. 293T (human kidney epithelial cells) and CHO (Chinese hamster ovary cells) cells were grown in DMEM (GibcoBRL Invitrogen) medium supplemented as for RPMI medium.
Adult peripheral blood samples, obtained from healthy adult donors after informed consent, were collected in acid citrate dextrose (ACD)–containing tubes. Peripheral blood mononuclear cells (PBMCs) were isolated upon a Ficoll-Hypaque (Sigma-Aldrich, St Louis, MO) separation and to obtain peripheral blood leukocytes (PBLs), cells were adhered for 2 hours at 37°C. CD3+ T cells were purified by negative selection using the Rosette tetrameric complex system (StemCell Technologies, Vancouver, BC). The purity of cell isolation was monitored by fluorescence-activated cell sorting (FACS).
Transduction assays
PBLs and T lymphocytes were cultured in RPMI 1640 medium (GibcoBRL Invitrogen) supplemented with 10% FCS and penicillin/streptomycin. PBLs were prestimulated with anti-CD3 and anti-CD28 antibodies (1 μg/mL) in presence of hIL-2 (1 ng/mL) as described previously,15 whereas CD3+ cells were prestimulated for 3 days with rIL-7 (10 ng/mL; BD Biosciences). Briefly, 5E4 cells were seeded in 24-well plates and concentrated vector supernatants were added at MOIs ranging from 0.1 to 30. The percentage of GFP+ cells was determined by FACS 72 hours after transduction. Incubation with 10 μM AZT 2 hours prior to transduction was performed as described.38
For CD46- and/or SLAM receptor–blocking experiments, 5E4 cells (cell lines or primary T cells) were seeded in 24-well plates and were incubated for 2 hours with anti-CD46 and/or anti-SLAM antibodies prior to infection. Concentrated viral supernatants were added to the cells at MOI = 1 or 10 for cell lines or primary T cells, respectively.
CD46 knockdown
Briefly, 293T cells were transduced with LVs encoding the shRNA183 and shRNA186 (Sigma-Aldrich). Transduced cells were selected by the addition of puromycin to the culture medium (1 mg/mL). These cells were used as target cells for transduction with the different MV-gp–pseudotyped LVs, and CD46 down-regulation was measured by FACS with anti–CD46-FITC mAb (BD Biosciences).
Binding assays
1E6 293T or CHO cells were washed with binding buffer (PBS/2% FCS/0.1% azide) and subsequently incubated with 60 μL low-speed concentrated viral supernatant for 1 hour at 4°C. After washing the cells, vector binding was revealed by incubation with anti-H (Y55) or anti-F (Y503) antibodies for 1 hour at 4°C followed by 2 washes in binding buffer and incubation with APC-mAb (BD Biosciences) was performed (1 hour, 4°C) followed by a wash step. Vector-cell binding was determined by FACS.
Cell-cell fusion assays
Briefly, cells were seeded onto 10-cm dishes and cotransfected with p8.91, HIV-SFFV-GFP, pCMV-RDTR, and corresponding MV-gp encoding plasmids as described before. Fluorescence images of vector-producing cells were taken using a Zeiss microscope (Carl Zeiss, Jena, Germany) model AxioVert 25, magnification 40×/0.55 Ph2. Pictures were taken with a Canon PowerShot digital camera (Canon, Tokyo, Japan) equipped with a Zeiss adapter tube for camera (Carl Zeiss). Images of vector-producing cells were acquired with IPhoto version 2.0.1 software for Mac OS X.3 (Apple, Cupertino, CA). Twenty-four or 48 hours after transfection, cells were fixed with glutaraldehyde and stained with May-Grünwald and Giemsa solutions (Merck, West Point, PA) according to the manufacturer's recommendations.
Intracellular cytokine staining
Intracellular cytokine staining was performed as previously described.15
PY/7-AAD cell-cycle analysis
Cell-cycle analysis was performed by staining DNA and RNA with 7-amino-actinomycin-D (7AAD) and pyronin Y, respectively, as previously described.15
Results
Efficient pseudotyping of MLV and HIV vectors with measles virus H and F gps
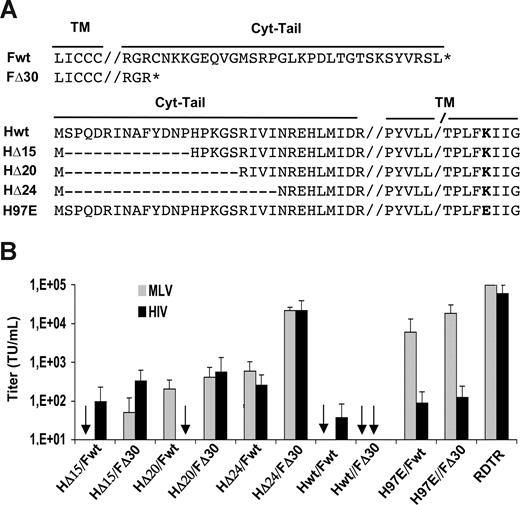
Although the factors that limit formation of infectious vector pseudotypes are not fully understood, the length of the cytoplasmic tail and the composition of the transmembrane components of several glycoproteins seem to play an important role.33,35,39,40 Indeed, pseudotyping of MLV or HIV vectors with Edmonston strain wild-type MV hemagglutinin (Hwt) responsible for receptor binding, and the wild-type fusion glycoprotein (Fwt), responsible for cell-cell and virus-cell fusion, was highly inefficient. We obtained no infectious titers at all for MLV vectors and extremely low titers for HIV vectors with these unmodified MV-gps (Figure 1A,B).
Efficient pseudotyping of MLV- and HIV-derived vectors with wild-type or mutant F and H MV-gps. (A) Schematic presentation of wild-type or mutant F and H gps from the Edmonston MV strain. The double vertical lines (//) separate the transmembrane sequences (TM) from the cytoplasmic domain (cyt-tail). The single line (/) indicates that part of TM is not shown. HΔ15, HΔ20, and HΔ24 represent H mutants with progressive deletions of the cytoplasmic tail. H97E contains a single amino acid mutation at position 97 in the TM region of wt H. FΔ30 contains only 3 residual amino acids of the F wt cytoplasmic tail. (B) 293T cells were incubated with serial dilutions of GFP-encoding MLV or HIV vectors that were pseudotyped with Hwt, HΔ15, HΔ20, HΔ24, or H97E in combination with Fwt or FΔ30. Control incubations were performed with RDTR gp–pseudotyped vectors. Cells were analyzed for GFP expression 72 hours after transduction by FACS analysis. Titers are expressed as TU/mL. Data are shown as means plus or minus SD; n = 6.
Efficient pseudotyping of MLV- and HIV-derived vectors with wild-type or mutant F and H MV-gps. (A) Schematic presentation of wild-type or mutant F and H gps from the Edmonston MV strain. The double vertical lines (//) separate the transmembrane sequences (TM) from the cytoplasmic domain (cyt-tail). The single line (/) indicates that part of TM is not shown. HΔ15, HΔ20, and HΔ24 represent H mutants with progressive deletions of the cytoplasmic tail. H97E contains a single amino acid mutation at position 97 in the TM region of wt H. FΔ30 contains only 3 residual amino acids of the F wt cytoplasmic tail. (B) 293T cells were incubated with serial dilutions of GFP-encoding MLV or HIV vectors that were pseudotyped with Hwt, HΔ15, HΔ20, HΔ24, or H97E in combination with Fwt or FΔ30. Control incubations were performed with RDTR gp–pseudotyped vectors. Cells were analyzed for GFP expression 72 hours after transduction by FACS analysis. Titers are expressed as TU/mL. Data are shown as means plus or minus SD; n = 6.
Consequently, we tested a panel of H MV-gps in which the cytoplasmic tail was truncated at different positions (HΔ15, HΔ20, HΔ24), for pseudotyping of both MLV- and HIV-derived vectors. A schematic diagram of these cytoplasmic tail mutants is presented in Figure 1A. Since one of the hurdles in efficient pseudotyping of retroviral vectors with Hwt and Fwt might be the extreme high syncytia formation in the producer cells induced by H/F expression, we also included the H97E mutant gp (Figure 1A). This mutant is, in the context of recombinant MV, fully capable of infecting target cells (virus-to-cell fusion) but is not able to induce syncytia (cell-to-cell fusion) (S.J.R., L. Hallak, and C. Hu, unpublished data, February 2007). In addition, we included one mutant of the F glycoprotein, which carries a truncated cytoplasmic tail of only 3-aa's long (FΔ30; Figure 1A). We tested Hwt and all these H mutants in combination with Fwt or FΔ30 for MLV and HIV vector pseudotyping. As a control, we used the RDTR gp, which allows efficient lentiviral pseudotyping.35
Pseudotyping of MLV vectors with the H97E gp in combination with Fwt or FΔ30 was highly efficient, leading to a 1000-fold increase of titer compared with Fwt/Hwt MLV pseudotypes (Figure 1B). Surprisingly, the effect on titer improvement was minor for this combination of gps when used to pseudotype HIV vectors. Although we had an increase in titer for MLV as well as for HIV vectors with the HΔ15 and HΔ20 mutants, compared with Hwt, only the mutant HΔ24, in combination with FΔ30, resulted in efficient pseudotyping of MLV vectors (Figure 1B). More importantly, HIV vectors pseudotyped with FΔ30 and HΔ24 yielded titers of up to 1E5 TU/mL versus 8E2 TU/mL obtained for Hwt/Fwt-pseudotyped HIV vectors.
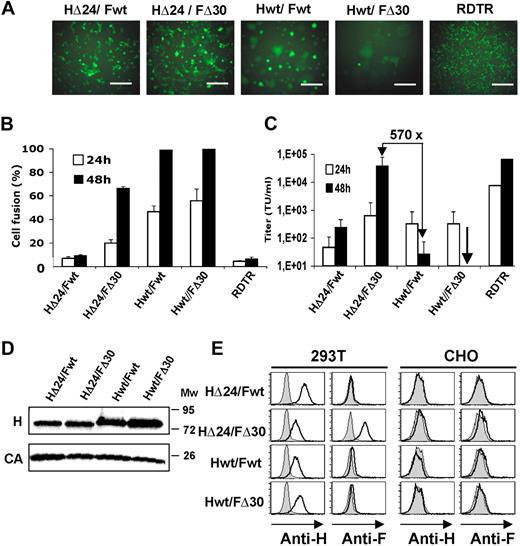
HΔ24/FΔ30-pseudotyped HIV vectors showed reduced cell fusion in producer cells
Since 293T producer cells abundantly express hCD46, one of the MV receptors, expression of H and F in these cells leads to syncytia and high cytotoxicity, which might interfere with vector assembly and release. Indeed, as expected, Fwt or FΔ30 transfected into 293T producer cells in the absence of H was not able to mediate cell fusion, whereas both proteins in the presence of Hwt induced strong cell-to-cell fusion already at 24 hours (Figure 2A). 293T cells were fixed and stained with Giemsa staining solution to determine the fusion index, which reached 100% at 48 hours after transfection with Hwt/Fwt and Hwt/FΔ30 (Figure 2B). Next, we assessed the competence of truncated HΔ24 protein to support F-mediated cell-to-cell and virus-to-cell fusion. HΔ24 cotransfected with Fwt did not support prominent syncytium formation as detected for Hwt, and the HΔ24/Fwt combination gave only a slight increase in lentivector titer compared with Hwt/Fwt HIV pseudotyping (Figure 2B,C).
Codisplay of cytoplasmic tail deletion mutants of H and F gps on HIV vectors results in high titers. 293T cells were transfected with HIV-gagpol, HIV-GFP vector, and the combination of H and F gps: Hwt/Fwt, HΔ24/FΔ30, Hwt/FΔ30, and HΔ24/Fwt. RDTR was used as a control gp. (A) Images show GFP expression and syncytia formation of 293T producer cells 24 hours after transfection. Scale bar represents 1000 μm. (B) At 24 and 48 hours after transfection, cells were fixed and stained with Giemsa staining solution, and syncytia formation (percentage cell fusion) was quantified. (C) 293T cells were incubated with serial dilutions of GFP-encoding HIV vectors pseudotyped with Hwt/Fwt, HΔ24/FΔ30, Hwt/FΔ30, HΔ24/Fwt, or RDTR gps. Vector supernatant was harvested at 24 or 48 hours after transfection. Cells were analyzed for GFP expression 72 hours after transduction by FACS. Titers are expressed as TU/mL (means ± SD; n = 6). (D) HIV-GFP vectors were pseudotyped with Hwt/Fwt, HΔ24/FΔ30, Hwt/FΔ30, and HΔ24/Fwt and purified over a sucrose cushion. Subsequently, viral gps, Hwt and HΔ24, were detected by Western blot with an antibody recognizing H ecto-domain; incubation with an anti-HIV p24 antibody was used to reveal HIV capsid (CA). (E) HIV-GFP vectors were pseudotyped with Hwt/Fwt, HΔ24/FΔ30, Hwt/FΔ30, and HΔ24/Fwt. A cell-binding assay was performed using the different vector preparations, concentrated by low-speed centrifugation. Both 293T cells (CD46+, SLAM−) and control CHO cells (CD46−, SLAM−) were incubated with the indicated HIV vector pseudotypes for 1 hour. After washing, bound viral particles were detected using conformational antibodies directed against the ectodomain of H gp (anti-H) or F gp (anti-F). Data shown are representative of 3 experiments.
Codisplay of cytoplasmic tail deletion mutants of H and F gps on HIV vectors results in high titers. 293T cells were transfected with HIV-gagpol, HIV-GFP vector, and the combination of H and F gps: Hwt/Fwt, HΔ24/FΔ30, Hwt/FΔ30, and HΔ24/Fwt. RDTR was used as a control gp. (A) Images show GFP expression and syncytia formation of 293T producer cells 24 hours after transfection. Scale bar represents 1000 μm. (B) At 24 and 48 hours after transfection, cells were fixed and stained with Giemsa staining solution, and syncytia formation (percentage cell fusion) was quantified. (C) 293T cells were incubated with serial dilutions of GFP-encoding HIV vectors pseudotyped with Hwt/Fwt, HΔ24/FΔ30, Hwt/FΔ30, HΔ24/Fwt, or RDTR gps. Vector supernatant was harvested at 24 or 48 hours after transfection. Cells were analyzed for GFP expression 72 hours after transduction by FACS. Titers are expressed as TU/mL (means ± SD; n = 6). (D) HIV-GFP vectors were pseudotyped with Hwt/Fwt, HΔ24/FΔ30, Hwt/FΔ30, and HΔ24/Fwt and purified over a sucrose cushion. Subsequently, viral gps, Hwt and HΔ24, were detected by Western blot with an antibody recognizing H ecto-domain; incubation with an anti-HIV p24 antibody was used to reveal HIV capsid (CA). (E) HIV-GFP vectors were pseudotyped with Hwt/Fwt, HΔ24/FΔ30, Hwt/FΔ30, and HΔ24/Fwt. A cell-binding assay was performed using the different vector preparations, concentrated by low-speed centrifugation. Both 293T cells (CD46+, SLAM−) and control CHO cells (CD46−, SLAM−) were incubated with the indicated HIV vector pseudotypes for 1 hour. After washing, bound viral particles were detected using conformational antibodies directed against the ectodomain of H gp (anti-H) or F gp (anti-F). Data shown are representative of 3 experiments.
An intermediate fusion phenotype was observed after HΔ24/FΔ30 transfection, which induced only 20% of cell fusion at 24 hours versus 40% to 50% for Hwt/Fwt. At the time point of maximum vector production (48 hours; Figure 2C), the HΔ24/FΔ30 vector producer cell never reached 100% of cell fusion as seen for Hwt/Fwt or Hwt/FΔ30 (Figure 2B). In addition, as indicated in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article), only the HΔ24/FΔ30-LVs were efficiently concentrated (100-fold) to a titer of 6E6 TU/mL, comparable with titers obtained for RDTR-pseudotyped LVs.
Thus, replacing Fwt by FΔ30 compensated the nonfusogenic phenotype of the combination HΔ24/Fwt to some extent, which might indicate the restoration of a functional H/F complex and, importantly, resulted in infectious HIV vector particles.
Cytoplasmic tail deletion mutants of H and F gps are efficiently coincorporated on LV particles
An LV will give titer only when both H and F gps are efficiently incorporated on the vector surface since H is indispensable for binding of the vector particle to the MV receptors, CD46 and/or SLAM, and subsequently F is needed to induce the virus-cell fusion step.
We first confirmed efficient incorporation of Hwt and HΔ24 for all combinations of vector pseudotypes, Hwt/Fwt, HΔ24/FΔ30, Hwt/FΔ30, and HΔ24/Fwt by Western blot (Figure 2D). Further, we assessed whether H and F gps were incorporated in the same LV particle by performing a virus-cell binding assay. As target cells, we used 293T cells that express CD46. CHO cells were used as a negative control since they do not express CD46 or SLAM receptors.41 Viral supernatants containing Hwt/Fwt-LVs, HΔ24/FΔ30-LVs, Hwt/FΔ30-LVs, and HΔ24/Fwt-LVs were concentrated by low-speed centrifugation (Table S1). The cells were incubated with the concentrated viral particles at 4°C for 1 hour. The resultant binding of H and F on living cells was determined by H- and F-specific antibodies. As expected, the presence of H or F was not detected on the surface of control CHO cells (Figure 2E), showing that the Hwt/Fwt-LVs, HΔ24/FΔ30-LVs, Hwt/FΔ30-LVs, and HΔ24/Fwt-LVs did not bind to their cell surface. In contrast, H was readily detected at the cell surface of CD46+ 293T cells for the 4 different LV pseudotypes (Figure 2E). However, only for the HΔ24/FΔ30-pseudotyped vector particles did we detect a shift in F marking by FACS analysis (Figure 2E). In conclusion, efficient coincorporation of H and F MV-gps occurred only for the combination of HΔ24/FΔ30, which resulted in the active conformation of H/F complexes allowing efficient virus-cell fusion.
HΔ24/FΔ30–pseudotyped lentivectors conserve the parental MV tropism
The cytoplasmic tail truncated H and F mutants, HΔ24 and FΔ30, are derived from the gps from the Edmonston vaccine strain of MV, which initiates infections through hCD46, ubiquitously expressed on human nucleated cells,27,28 and through SLAM, present on dendritic cells B and T lymphocytes.25,26
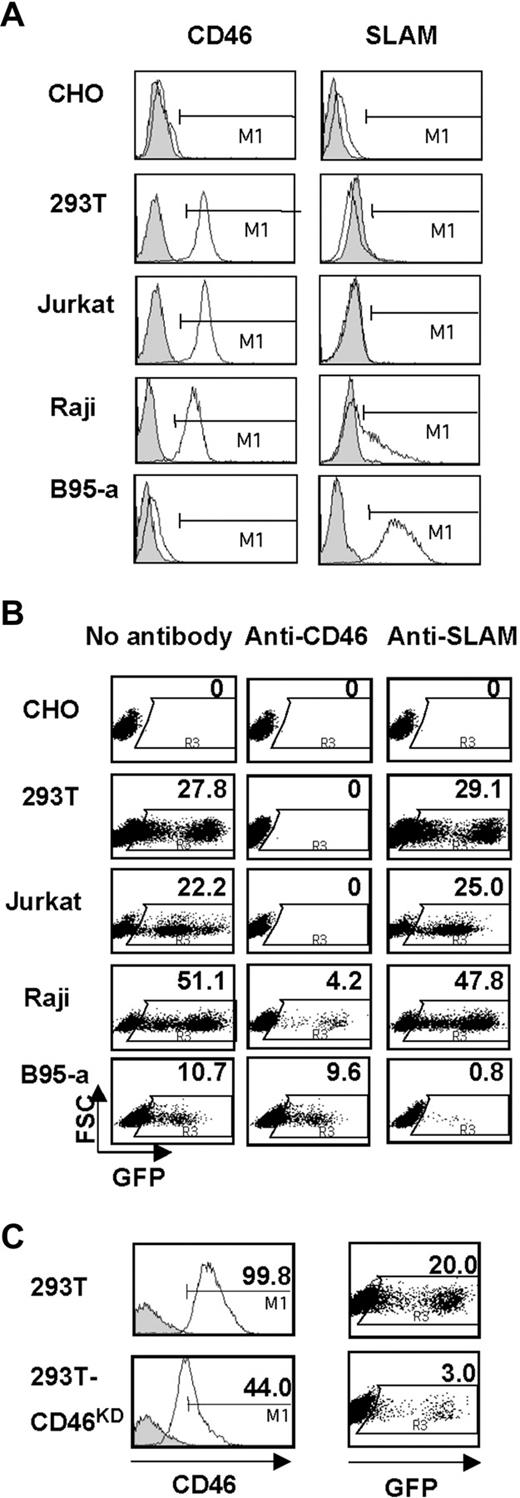
The HΔ24/FΔ30-pseudotyped lentivectors efficiently transduced the CD46+/SLAM− 293T and Jurkat cell lines, whereas they did not transduce CHO cells lacking both receptors (Figure 3A,B). To demonstrate that transduction occurred specifically through the CD46 receptor, we incubated 293T and Jurkat cells with an anti-CD46–blocking antibody (mAb 13/46). As shown in Figure 3B, specific blocking of CD46 receptor completely abolished transduction with HΔ24/FΔ30-LVs. The specificity of blocking was confirmed by incubation with anti-SLAM antibody, which did not affect HΔ24/FΔ30-LVs transduction levels on 293T and Jurkat target cells. Moreover, down-regulation of hCD46 expression in 293T target cells by shRNA knockdown reduced the HΔ24/FΔ30-LV transduction levels by 7-fold (Figure 3C), confirming that CD46 was used as entry receptor for virus-cell fusion. Since the alternative MV receptor, SLAM, is present on primary T cells and B cells, we tested HΔ24/FΔ30-LV entry through SLAM. Indeed, blocking of CD46 receptor binding in CD46+/SLAM+ Raji cells prior to HΔ24/FΔ30-LV transduction resulted in a residual transduction. Moreover, the B95a cell line, expressing SLAM but not CD46, was transduced to high levels by the MV-gp–pseudotyped LVs (Figure 3A-B). Of importance, blocking of SLAM with anti-SLAM antibody (IPO-3) reduced transduction by 90%, indicating specific use of SLAM for vector entry in B95-a cells, whereas incubation with anti-CD46 antibody did not significantly reduce HΔ24/FΔ30-LV transduction (Figure 3B).
HΔ24/FΔ30-pseudotyped LVs conserve the tropism of Edmonston MV strain. (A) SLAM and CD46 surface staining (open histograms) of the different cell lines (CHO, 293T, Jurkat, Raji, and B95a). Closed histograms represent staining with the isotype IgG control. (B) The different cell lines were transduced with a HΔ24/FΔ30-pseudotyped HIV-GFP vector at an MOI of 1 in the absence of antibody (no antibody) or after 2-hour incubation with an anti-CD46 antibody that blocks H binding to CD46 receptor or, as control, with anti-SLAM antibody prior to transduction. In the case of B95-a cells, a specific anti-SLAM antibody was used to block the SLAM receptor, whereas anti-CD46 antibody was used as a control prior to transduction. Cells were analyzed for GFP expression 72 hours after transduction by FACS analysis. Data shown here are representative of 4 independent experiments. (C) 293T cells were transduced with 2 different shRNA LVs to knock down hCD46 (293T-CD46KD). Open histograms represent the expression of CD46 in 293T or 293T-CD46KD. Closed histograms represent staining with the isotype IgG control. Both cell lines were subsequently transduced with HΔ24/FΔ30-pseudotyped HIV-GFP vector at MOI = 1. Dot blots represent the percentage of GFP+ cells 3 days after transduction, as analyzed by FACS.
HΔ24/FΔ30-pseudotyped LVs conserve the tropism of Edmonston MV strain. (A) SLAM and CD46 surface staining (open histograms) of the different cell lines (CHO, 293T, Jurkat, Raji, and B95a). Closed histograms represent staining with the isotype IgG control. (B) The different cell lines were transduced with a HΔ24/FΔ30-pseudotyped HIV-GFP vector at an MOI of 1 in the absence of antibody (no antibody) or after 2-hour incubation with an anti-CD46 antibody that blocks H binding to CD46 receptor or, as control, with anti-SLAM antibody prior to transduction. In the case of B95-a cells, a specific anti-SLAM antibody was used to block the SLAM receptor, whereas anti-CD46 antibody was used as a control prior to transduction. Cells were analyzed for GFP expression 72 hours after transduction by FACS analysis. Data shown here are representative of 4 independent experiments. (C) 293T cells were transduced with 2 different shRNA LVs to knock down hCD46 (293T-CD46KD). Open histograms represent the expression of CD46 in 293T or 293T-CD46KD. Closed histograms represent staining with the isotype IgG control. Both cell lines were subsequently transduced with HΔ24/FΔ30-pseudotyped HIV-GFP vector at MOI = 1. Dot blots represent the percentage of GFP+ cells 3 days after transduction, as analyzed by FACS.
Summarizing, the H and F mutant gps, HΔ24 and FΔ30, respectively, efficiently pseudotype LVs that conserve virus-cell entry through the MV receptors, hCD46 and hSLAM.
HΔ24/FΔ30-LVs are superior to VSV-G-LVs for the transduction of primary T cells
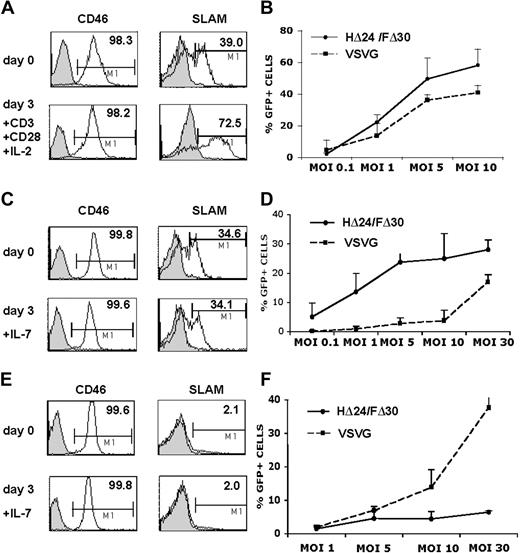
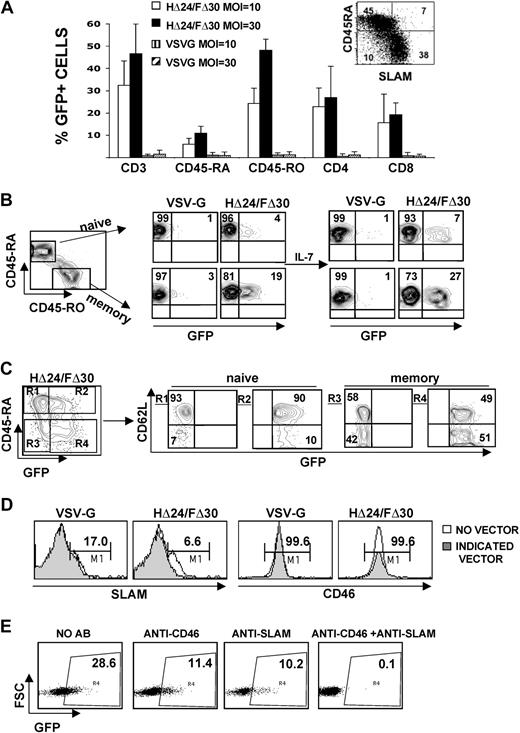
First we evaluated the capacity of HΔ24/FΔ30-LVs to transduce T lymphocytes. Since it is generally accepted that quiescent T cells are not permissive to LVs, we prestimulated peripheral blood lymphocytes (PBLs) through the TCR by incubation with anti-CD3 and anti-CD28 antibodies. This resulted in a high up-regulation of SLAM receptor, whereas the CD46 expression levels remained unchanged compared with unstimulated cells (Figure 4A). We incubated the prestimulated PBLs with escalating doses of HΔ24/FΔ30-LVs or VSV-G-LVs. Even at a low MOI of 1, the HΔ24/FΔ30-LV was able to transduce 22% of the PBLs (Figure 4B).
HΔ24/FΔ30-displaying LVs outperform VSV-G-LVs for the transduction of IL-7–prestimulated adult T cells. (A) Surface expression of SLAM or CD46 receptors on unstimulated PBLs or on PBLs stimulated for 3 days with anti-CD3+ anti-CD28 antibodies in the presence of IL-2. (B) PBLs were stimulated for 3 days with anti-CD3 and anti-CD28 antibodies in the presence of IL-2 and subsequently transduced with HΔ24/FΔ30- or VSV-G–pseudotyped HIV-GFP vectors at the indicated vector doses. Transduced cells were kept in culture for 3 days in the presence of IL-2 and the percentage of GFP expression was monitored by FACS (means ± SD; n = 3). (C,E) The surface expression of SLAM or CD46 receptors on resting adult or cord blood CD3+ T cells, respectively, immediately upon isolation by negative antibody-mediated selection or upon incubation of these T cells with rIL-7 for 3 days. Purified resting adult (D) or cord blood (F) CD3+ T cells were stimulated for 3 days with rIL-7 and subsequently transduced with HΔ24/FΔ30- or VSV-G–pseudotyped HIV-GFP vectors in the presence of rIL-7 at the indicated vector doses. Transduced cells were maintained in culture for 3 days and GFP expression was then monitored by FACS analysis (means ± SD; n = 5).
HΔ24/FΔ30-displaying LVs outperform VSV-G-LVs for the transduction of IL-7–prestimulated adult T cells. (A) Surface expression of SLAM or CD46 receptors on unstimulated PBLs or on PBLs stimulated for 3 days with anti-CD3+ anti-CD28 antibodies in the presence of IL-2. (B) PBLs were stimulated for 3 days with anti-CD3 and anti-CD28 antibodies in the presence of IL-2 and subsequently transduced with HΔ24/FΔ30- or VSV-G–pseudotyped HIV-GFP vectors at the indicated vector doses. Transduced cells were kept in culture for 3 days in the presence of IL-2 and the percentage of GFP expression was monitored by FACS (means ± SD; n = 3). (C,E) The surface expression of SLAM or CD46 receptors on resting adult or cord blood CD3+ T cells, respectively, immediately upon isolation by negative antibody-mediated selection or upon incubation of these T cells with rIL-7 for 3 days. Purified resting adult (D) or cord blood (F) CD3+ T cells were stimulated for 3 days with rIL-7 and subsequently transduced with HΔ24/FΔ30- or VSV-G–pseudotyped HIV-GFP vectors in the presence of rIL-7 at the indicated vector doses. Transduced cells were maintained in culture for 3 days and GFP expression was then monitored by FACS analysis (means ± SD; n = 5).
However, it has become clear now that TCR activation of T cells impairs their half-life, and results in inversion of CD4/CD8 ratio, loss of naive T-cell subsets, skews in TCR repertoire, and reduced immunocompetence.15,16 We and others have shown previously that IL-7 prestimulation of resting T cells renders them permissive to VSV-G-LVs at high MOI without inducing a switch from naive to memory phenotype.14,15,17 Therefore, we compared VSV-G-LVs and HΔ24/FΔ30-LVs for their capacity to transduce rIL-7–prestimulated highly purified adult T cells. Of note, the surface expression of CD46 and SLAM after 3 days of rIL-7 prestimulation was equivalent to that observed on unstimulated quiescent CD3+ T cells (Figure 4C). The IL-7–prestimulated CD3+ adult T cells were subsequently transduced with HΔ24/FΔ30 and VSV-G–pseudotyped LVs at escalating vector doses. As expected, transduction with VSV-G-LVs was very low, even at an MOI of 10, not exceeding 3% of transduction (Figure 4D). An MOI of at least 20 to 30 was needed for efficient VSV-G-LV transduction (Figure 4D) of IL-7–prestimulated T cells, in agreement with previous results.15 In contrast, the HΔ24/FΔ30-LVs transduced IL-7–prestimulated adult T cells efficiently at 20- to 30-fold lower vector doses (MOI = 1; Figure 4D). At a high MOI of 10, the HΔ24/FΔ30-LVs outperformed the VSV-G-LVs by far, allowing on average 25% to 30% transduction after a single exposure to the vector. To exclude an effect of the HΔ24/FΔ30-LVs on the cell-cycle status of the IL-7–prestimulated T cells, we assessed the cell-cycle entry immediately prior to and at 72 hours after transduction. Importantly, the HΔ24/FΔ30-LVs did not induce more cell-cycle entry of IL-7–prestimulated adult cells compared with incubation with no vector at all or with VSV-G-LVs (Figure S1). Since binding to SLAM as well as to CD46 could lead to activation of T cells,25,26,42,43 we sought to verify whether HΔ24/FΔ30-LVs did not induce naive to memory phenotype switch of the transduced IL-7–prestimulated T cells. Memory T cells were transduced with HΔ24/FΔ30-LVs at high levels, ranging from 30% to 50% transduction at MOI = 10 (mean, 39.2% ± 9.3%), resulting in 15- to 20-fold higher transduction than with VSV-G-LVs (mean, 2.4% ± 0.3%). In contrast, naive T cells were transduced to a significantly lower extent (mean, HΔ24/FΔ30 = 11.2% ± 7.3%; VSV-G = 0.9% ± 0.1%) but maintained their naive phenotype. In general, CD4+ cells were transduced more efficiently with HΔ24/FΔ30-LVs than CD8+ cells (means, CD4+ cells: HΔ24/FΔ30 = 24.3% ± 4.2%; VSV-G = 1.5% ± 0.1% versus CD8+ cells: HΔ24/FΔ30 = 15.1% ± 5.3%; VSV-G = 1.0% ± 0.1%). Of note, SLAM expression was down-regulated on IL-7–prestimulated adult CD3 T cells after incubation with HΔ24/FΔ30-LVs, whereas this was not the case for VSV-G-LVs (data not shown).
To further assess the capacity of the HΔ24/FΔ30-LVs to transduce naive T cells, we transduced cord blood (CB) T cells, which contain 90% CD45RA+ immature T cells, after a 3-day stimulation with rIL-7. Surprisingly, this resulted in low transduction of these cells by HΔ24/FΔ30-LVs even at high vector doses (MOI = 30) compared with VSV-G-LVs at similar MOI (Figure 4F). In agreement with previous data,10,15 rIL-7–prestimulated CB CD3+ T cells were highly permissive to high doses of VSV-G-LVs (Figure 4F). Although more than 20% of these IL-7–prestimulated CB T cells were strongly cycling, they were not efficiently transduced by HΔ24/FΔ30-LVs. This vector did not induce higher cell-cycle entry compared with no vector or VSV-G-LV incubation (Figure 4F; Figure S1). Of note, SLAM receptor was hardly detected on the surface of quiescent or IL-7–prestimulated CB T cells, whereas CD46 expression remained high (Figure 4E).
In conclusion, these results demonstrate that IL-7–prestimulated adult T cells are highly susceptible to the HΔ24/FΔ30-LV at low vector doses, at which VSV-G-LVs do not transduce these cells.
The HΔ24/FΔ30-LV pseudotypes permit efficient transduction of quiescent T cells
Since down-regulation of SLAM was detected on IL-7–prestimulated adult T cells upon incubation with the HΔ24/FΔ30-LVs, we hypothesized that signaling induced through SLAM might be sufficient to allow lentiviral transduction of resting T cells in absence of exogenous stimulation.
Quiescent adult CD3+ T cells were purified by negative antibody-mediated selection to not activate them during isolation. We next incubated the resting T cells with HΔ24/FΔ30- or VSV-G–pseudotyped LVs. Importantly, the HΔ24/FΔ30-LVs were able to efficiently transduce resting adult CD3+ T cells (25%-40%) in the absence of any stimulus, following a single exposure with this vector (MOI = 10; Figure 5A). In agreement with previous reports,10,15 at an MOI of 30, the VSV-G-LVs were unable to transduce unstimulated resting T cells (Figure 5A). A detailed analysis of the transduction levels for the T-cell subsets is shown in Figure 5A. Both CD4+ and CD8+ T cells were transduced by HΔ24/FΔ30-LVs with a preference for the CD4+ subset (Figure 5A). As shown in Figure 5A and B, HΔ24/FΔ30-LVs were able to transduce both resting naive and memory adult T cells, whereas these cells remained refractory to VSV-G-LV transduction. Increasing the vector doses from MOI = 10 to MOI = 30 for HΔ24/FΔ30-LVs only slightly increased naive T-cell transduction, whereas memory cell transduction was significantly increased, revealing a preference of this vector to transduce the memory subset (Figure 5A). Of note, SLAM is expressed on 80% of the memory subset (Figure 5A inset).
The HΔ24/FΔ30-displaying LVs efficiently transduce quiescent adult T cells. (A) Highly purified resting adult T cells were isolated by negative antibody-mediated selection to avoid cell activation and then transduced with HΔ24/FΔ30- or VSV-G–pseudotyped HIV-GFP vectors at an MOI of 10 or 30 in the absence of exogenous stimuli. Three days after transduction, surface staining for naive and memory subsets was performed by anti-CD45RA/anti-CD45RO double staining. In parallel, surface staining for the CD3+, CD4+, and CD8+ T-cell subsets was performed and the percentage of GFP expression for each of these T-cell subsets was analyzed by FACS (means ± SD; n = 5). Inset shows CD45RA versus SLAM expression on freshly isolated total CD3+ T cells (B) Resting T cells were transduced with HΔ24/FΔ30- or VSV-G–pseudotyped LVs at an MOI of 10. After 3 days, part of the transduced cell cultures was continued in the presence of rIL-7 for 3 more days to verify stable GFP expression. For both culture conditions, surface staining for naive and memory subsets was performed by anti-CD45RA/anti-CD45RO double staining. Dot blots represent the percentage of GFP+ cells in the CD45RA+ naive T cells (top row) and the percentage GFP+ cells in the CD45RO+ memory T cells (bottom row). Data are representative of 6 experiments. (C) Resting T cells were transduced with HΔ24/FΔ30- or VSV-G–pseudotyped LVs at an MOI of 30. Surface staining for naive T cells was performed by anti-CD45RA staining. Dot blot represents the GFP+ CD45RA+ naive T cells (top right quadrant R2) and the GFP+ CD45RA− memory T cells (bottom right quadrant R4). For each gate, R1, R2, R3, and R4, the expression of CD62L is shown. Data are representative of 3 experiments. (D) Surface expression of SLAM or CD46 receptors on resting adult CD3+ cells after transduction with HΔ24/FΔ30- or VSV-G–pseudotyped lentivectors at an MOI of 10. (E) Resting adult T cells were incubated with or without anti-SLAM or anti–CD46 receptor–blocking antibodies for 3 hours and subsequently transduced with HΔ24/FΔ30-LVs or VSV-G-LVs at an MOI of 10. The percentage of GFP+ cells was analyzed by FACS 3 days after transduction.
The HΔ24/FΔ30-displaying LVs efficiently transduce quiescent adult T cells. (A) Highly purified resting adult T cells were isolated by negative antibody-mediated selection to avoid cell activation and then transduced with HΔ24/FΔ30- or VSV-G–pseudotyped HIV-GFP vectors at an MOI of 10 or 30 in the absence of exogenous stimuli. Three days after transduction, surface staining for naive and memory subsets was performed by anti-CD45RA/anti-CD45RO double staining. In parallel, surface staining for the CD3+, CD4+, and CD8+ T-cell subsets was performed and the percentage of GFP expression for each of these T-cell subsets was analyzed by FACS (means ± SD; n = 5). Inset shows CD45RA versus SLAM expression on freshly isolated total CD3+ T cells (B) Resting T cells were transduced with HΔ24/FΔ30- or VSV-G–pseudotyped LVs at an MOI of 10. After 3 days, part of the transduced cell cultures was continued in the presence of rIL-7 for 3 more days to verify stable GFP expression. For both culture conditions, surface staining for naive and memory subsets was performed by anti-CD45RA/anti-CD45RO double staining. Dot blots represent the percentage of GFP+ cells in the CD45RA+ naive T cells (top row) and the percentage GFP+ cells in the CD45RO+ memory T cells (bottom row). Data are representative of 6 experiments. (C) Resting T cells were transduced with HΔ24/FΔ30- or VSV-G–pseudotyped LVs at an MOI of 30. Surface staining for naive T cells was performed by anti-CD45RA staining. Dot blot represents the GFP+ CD45RA+ naive T cells (top right quadrant R2) and the GFP+ CD45RA− memory T cells (bottom right quadrant R4). For each gate, R1, R2, R3, and R4, the expression of CD62L is shown. Data are representative of 3 experiments. (D) Surface expression of SLAM or CD46 receptors on resting adult CD3+ cells after transduction with HΔ24/FΔ30- or VSV-G–pseudotyped lentivectors at an MOI of 10. (E) Resting adult T cells were incubated with or without anti-SLAM or anti–CD46 receptor–blocking antibodies for 3 hours and subsequently transduced with HΔ24/FΔ30-LVs or VSV-G-LVs at an MOI of 10. The percentage of GFP+ cells was analyzed by FACS 3 days after transduction.
Importantly, no changes in the total CD4+/CD8+ cell ratio, no loss of naive T-cell marker, and no skewing of the memory/naive subsets were observed after HΔ24/FΔ30-LV transduction. To rule out the possibility that HΔ24/FΔ30-LVs induced pseudotransduction only, we continued the culture of these quiescent transduced cells in the presence of rIL-7. FACS analysis 6 days after transduction proved that both the memory and naive cells were indeed stably transduced (Figure 5B). The HΔ24/FΔ30-LV–transduced naive T cells demonstrated high CD62L surface expression and did not lose the CCR7 surface marker, suggesting that they did not gain effector cell phenotype (Figure 5C and data not shown).
In addition, we incubated the resting T cells with HΔ24/FΔ30-LVs in the presence or absence of AZT, a reverse transcriptase (RT) inhibitor. RT inhibition resulted on average in 92% decrease in transduction efficiency (data not shown), hence confirming the true transduction of resting T cells (means: HΔ24/FΔ30 = 14.1% ± 1.1% and HΔ24/FΔ30 + AZT = 1.2% ± 0.1%).
To identify which receptor was used for HΔ24/FΔ30-LV entry and transduction of quiescent T cells, we revealed SLAM or CD46 surface expression on resting T cells incubated with HΔ24/FΔ30-LVs, VSV-G-LVs, or no vector. CD46 receptor expression was unaffected in all cases, whereas the natural MV receptor, SLAM, was down-regulated only after contact with HΔ24/FΔ30-LVs (Figure 5D). Subsequently, we blocked the SLAM and/or CD46 receptor by preincubation of resting T cells with specific antibodies before HΔ24/FΔ30-LV transduction. Transduction with HΔ24/FΔ30-LVs was blocked to the same levels with anti-SLAM or anti-CD46–blocking antibodies, whereas it was completely abolished when both blocking antibodies were added together (Figure 5E).
Overall, these results demonstrate that HΔ24/FΔ30-LVs are able to efficiently transduce quiescent adult T cells in the absence of any exogenous stimulus, thereby maintaining the appropriate CD4+/CD8+ ratios and conserving the memory and naive T-cell phenotypes.
Efficient transduction of quiescent T cells by the HΔ24/FΔ30-LVs does not induce cell-cycle progression or T-cell activation/differentiation
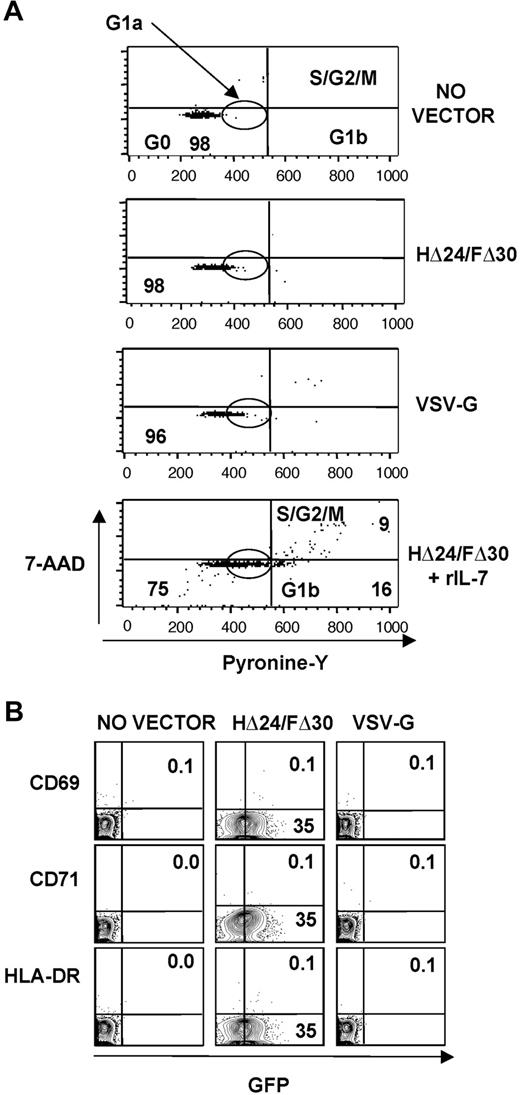
As transduction of peripheral blood T cells with LVs requires progression into the G1b phase of the cell cycle,10,13,15,17 it was important to determine whether transduction of quiescent adult CD3+ T cells by HΔ24/FΔ30-LVs induced cell-cycle entry. Using a method that allows simultaneous visualization of DNA (7-AAD staining) and RNA (PY staining) contents, we were able to distinguish among T cells residing in G0, G1a, G1b, S, and G2/M phases of the cell cycle (Figure 6A). Highly purified resting adult T cells were incubated with VSV-G-LVs or HΔ24/FΔ30-LVs (MOI = 10). As controls, cells were incubated with no vector or IL-7–prestimulated cells were transduced with HΔ24/FΔ30-LVs. Quiescent T cells incubated with VSV-G LVs remained in the G0/G1a phase of the cell cycle (> 96%; Figure 6A), resulting in very low transduction levels (< 3%; Figure 5A,B). As expected, transduction of IL-7–prestimulated cells with HΔ24/FΔ30-LVs induced cell-cycle entry into the G1b phase and S/G2/M phase of the cell cycle (Figure 6A), resulting in more than 20% transduction (Figure 4D). In contrast, quiescent T cells incubated with HΔ24/FΔ30-LVs remained in the G0/G1a phase of the cell cycle (> 98%) but resulted in high transduction levels (> 30%; Figure 5A,B). Of importance, some entry into the G1a phase of the cell cycle was detected after incubation of T cells with HΔ24/FΔ30-LVs (4% into G1a) and VSV-G LVs (10% into G1a) as evidenced by their increased RNA content in Figure 6A, compared with no vector incubation. In addition, we did not detect any up-regulation of early (CD69, CD25) or late (HLA-DR, CD71) activation markers in HΔ24/FΔ30-LV–transduced adult T cells. This expression profile was identical to that of quiescent T cells incubated with VSV-G-LVs or no vector (Figure 6B and data not shown).
Comparison of cell-cycle entry and activation of adult T cells following exposure to HΔ24/FΔ30-LVs or VSV-G-LVs. Resting adult CD3+ T cells were transduced with HΔ24/FΔ30- or VSV-G–displaying LVs, or no vector, and as a control IL-7–prestimulated T cells were transduced with HΔ24/FΔ30-displaying LVs. (A) Cell-cycle progression was monitored by simultaneously visualizing the RNA (pyronin-Y) and DNA (7-AAD) content of the T cells at day 3 of incubation. The percentages of cells in the G0/G1a, G1b, and S/G2/M phase of the cell cycle are indicated in the dot blots. (B) Surface staining for CD69, HLA-DR, and CD71 versus GFP+ cell expression is shown at day 3 after transduction. The percentage of activation marker–expressing GFP+ cells is indicated in the top right quadrant; the percentage of GFP+ cells not expressing the activation marker is indicated in the bottom right quadrant.
Comparison of cell-cycle entry and activation of adult T cells following exposure to HΔ24/FΔ30-LVs or VSV-G-LVs. Resting adult CD3+ T cells were transduced with HΔ24/FΔ30- or VSV-G–displaying LVs, or no vector, and as a control IL-7–prestimulated T cells were transduced with HΔ24/FΔ30-displaying LVs. (A) Cell-cycle progression was monitored by simultaneously visualizing the RNA (pyronin-Y) and DNA (7-AAD) content of the T cells at day 3 of incubation. The percentages of cells in the G0/G1a, G1b, and S/G2/M phase of the cell cycle are indicated in the dot blots. (B) Surface staining for CD69, HLA-DR, and CD71 versus GFP+ cell expression is shown at day 3 after transduction. The percentage of activation marker–expressing GFP+ cells is indicated in the top right quadrant; the percentage of GFP+ cells not expressing the activation marker is indicated in the bottom right quadrant.
T helper CD4+ T cells can be subdivided into 2 distinct subsets, Th1 and Th2, on the basis of their cytokine secretion pattern. It was reported that engagement of SLAM by an agonist antibody on activated T cells leads to IFN-γ production and directs cell differentiation toward the Th1 pathway.26,42 Since, in addition, SLAM is expressed at higher levels on Th1 than on Th2 cells,44 it was important to assess the effect of HΔ24/FΔ30-LV transduction on cytokine production of CD4+ T cells. We chose IFN-γ and IL-4 as representative cytokines for the Th1 and Th2 T-cell subsets, respectively.
We analyzed the CD4+ T cells incubated with either HΔ24/FΔ30-LVs or VSV-G-LVs (MOI = 10) or no vector for intracellular cytokine production. As expected, IL-2 was expressed by 30% of the CD4+ T cells, following 4 hours of PMA and ionomycin stimulation, regardless of whether the cells were transduced (Table 1). Of importance, only a minimal percentage (< 3%) of cells expressed IL-4 under either of these conditions. A small portion of IFN-γ–expressing cells was detected (10%; Table 1) upon HΔ24/FΔ30-LV or VSV-G-LV transduction. This IFN-γ–expressing profile was not significantly higher than observed in untransduced cells. Thus, these data demonstrate that the HΔ24/FΔ30-LVs do not induce skewing to either Th1 or Th2 phenotype.
Cytokine expression profile of LV-transduced CD4+ T cells
| Vector . | IL-2, % . | IFN-γ, % . | IL-4, % . |
|---|---|---|---|
| No vector | 24.2 ± 7.0 | 8.8 ± 0.4 | 1.8 ± 0.5 |
| HΔ24/FΔ30 | 22.3 ± 7.0 | 10.0 ± 0.1 | 2.2 ± 0.9 |
| VSV-G | 27.5 ± 9.5 | 12.1 ± 0.8 | 1.5 ± 0.6 |
| Vector . | IL-2, % . | IFN-γ, % . | IL-4, % . |
|---|---|---|---|
| No vector | 24.2 ± 7.0 | 8.8 ± 0.4 | 1.8 ± 0.5 |
| HΔ24/FΔ30 | 22.3 ± 7.0 | 10.0 ± 0.1 | 2.2 ± 0.9 |
| VSV-G | 27.5 ± 9.5 | 12.1 ± 0.8 | 1.5 ± 0.6 |
Quiescent CD4+ T cells were transduced with HΔ24/FΔ30 or VSV-G displaying LVs or incubated without vector and subsequently stimulated with PMA and ionomycin for 4 hours. Afterward, cells were permeabilized and incubated with anti–IL-2, anti–IL-4, or anti–IFN-γ sPE-coupled mAbs. As a control, an isotype PE–coupled mAb was used. Cells were washed, fixed, and analyzed by flow cytometry. The average of 3 experiments is shown (mean ±SD).
Overall, these results indicate that the HΔ24/FΔ30-LVs are capable of transducing quiescent T cells without the need to induce G1b phase cell-cycle entry and without changing their phenotype.
Discussion
This is the first demonstration of a new LV pseudotype that can efficiently transduce quiescent T cells in the absence of any other stimulus, and this without inducing cell-cycle progression at all. These vectors achieved 50% transduction of resting memory adult T cells as well as a significant proportion of naive adult T cells. In addition, the phenotype of the transduced T cells was conserved and in vitro cytokine profiling suggested that no skewing of T-cell subsets was detected.
This is also the first report demonstrating an efficient display of MV-gps H and F, on the surface of retroviral vectors. Only the combination of the cytoplasmic tail mutants, HΔ24 and FΔ30, resulted in high-titer MLV and HIV vectors of up to 1E7 TU/mL. Although in the context of wt and recombinant MV, cell-cell fusion and virus recovery are directly correlated,31 this was not a prerequisite in the context of an LV. Importantly, by combining the HΔ24 and FΔ30 gps, we demonstrated the efficient coincorporation of these gps on the LV surface in an active conformation allowing efficient virus-cell fusion. The Edmonston strain of MV initiates infection of host cells through CD46, expressed on all human nucleated cells,28 and through SLAM, present on dendritic cells, B and T lymphocytes.25,26 Indeed, the HΔ24/FΔ30-LVs conserved the parental tropism as confirmed by the transduction of CD46-expressing cell lines as well as cell lines expressing only SLAM. Although titers of HΔ24/FΔ30-LV are lower than VSV-G-LV titers, they can be concentrated up to 1E7 TU/mL, making their clinical application very attractive, especially since very low H/F-LV doses are needed to obtain similar or superior T-cell transduction levels compared with VSV-G-LVs.
We and others reported that transduction of peripheral blood T cells with VSV-G–pseudotyped LVs requires progression into the G1b phase of the cell cycle.11,13-15 Stimulation of T cells with cytokines such as IL-2 and IL-7 renders them permissive to lentiviral transduction in the absence of TCR activation. Interestingly, our novel H/F gp–displaying LVs were highly superior to the classical late-generation VSV-G-LVs since they transduced up to 35% of the IL-7–prestimulated T cells, whereas VSV-G-LVs did not allow transduction at equivalent vector doses. Interestingly, the novel HΔ24/FΔ30-pseudotyped LVs, in contrast to VSV-G-LVs, did not even require a IL-7 prestimulation since they efficiently transduced resting T cells without exogenous stimulation. Both SLAM and CD46 can act as costimulatory molecules for T-cell activation.26 We provide here clear evidence that the HΔ24/FΔ30-LVs efficiently transduced quiescent T cells without inducing G1b phase cell-cycle entry. This is in agreement with the fact that CD46 or SLAM engagement has no mitogenic effect on freshly isolated T cells.26,43 In addition, we did not reveal any induction of classical early or late activation markers upon HΔ24/FΔ30-LV transduction, where IL-7 stimulation slightly up-regulated these markers.10,15 Moreover, in vitro analysis of HΔ24/FΔ30-LV–transduced resting T cells showed no changes in cytokine profile, suggesting that T-cell populations were not skewed toward the Th0/Th1 phenotype as might have been expected from SLAM engagement,26 whereas IL-7 or IL-2 stimulation of T cells induces a slight bias toward Th1 phenotype.13 Preliminary results show that the HΔ24/FΔ30-LVs efficiently transduced not only quiescent T cells but also quiescent B-cells (E.V. and C.F., unpublished data, March 1, 2008, and Funke50 ), another important gene therapy target cell, refractory to transduction with VSV-G-LVs.45,46
Several hypotheses can be proposed to explain why H/F-LVs transduce completely quiescent cells. Binding of MV-gps to SLAM/CD46 receptors on quiescent cells could activate the uncoating process together with specific requirements for other steps of viral replication. Entry through SLAM/CD46 may also alter trafficking of the particles through cellular compartments, protecting them for proteasome degradation47 or inducing the uncoating process. In addition H/F-LVs might avoid interaction with postentry restriction factors using alternative cell entry mechanisms, for example, through SLAM/CD46. It will be of importance to elucidate the process facilitating quiescent T-cell transduction.
Interestingly, virus-cell entry into primary T cells seemed to occur primarily through SLAM and not through CD46 receptor. This hypothesis is supported by multiple findings. First, we obtained a high transduction level with HΔ24/FΔ30-LVs in IL-7–stimulated CD46+SLAM+ adult T cells, whereas CD46+SLAMlow CB naive T cells were barely transduced. Second, in TCR-stimulated T cells, which resulted in SLAM up-regulation, a higher transduction was observed. Third, we obtained a higher transduction of quiescent memory T cells compared with naive T cells, the latter subset expressing SLAM at low levels.26 We also detected a preferential transduction of CD4+ T cells, which express higher levels of SLAM than CD8+ cells.48 Finally, incubation of resting T cells with HΔ24/FΔ30-LVs resulted in SLAM down-regulation, whereas CD46 surface expression was unaffected. Figure 5E shows that anti-CD46 and anti-SLAM blockades are equally efficient in reducing HΔ24/FΔ30-LV transduction of resting T cells. Since Edmonston H gp binds more tightly to SLAM than to CD46,49 one possible hypothesis is that when SLAM is present, it will be preferentially used by H/F-LVs to enter the cells. However, once SLAM is blocked, the H/F-LV might use an alternative entry route, for example, through the CD46 receptor.
These novel H/F gp–displaying LVs will open the way to the design of refined gene transfer tools retargeted to specific cell types. In measles virus, the binding and fusion functions are separated into 2 proteins, H and F. This particular composition is advantageous for designing retargeted vectors without affecting fusion. Indeed, retargeted recombinant MVs are fully capable of fusion with the target cell membrane, indicating that change of receptor tropism does not interfere with F function.41 This makes H/F LVs good candidates for retargeting gene transfer to specific cell types, by insertion of specific ligands in H.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank A. Maisner for providing the H and F mutant expression plasmids and also C. Muller, R. Buckland, and D. Gerlier for providing antibodies.
This work was supported by grants from the Agence Nationale pour la Recherche contre le SIDA et les Hépatites Virales (ANRS, Paris, France), the Agence nationale de la Recherche (ANR, Paris, France), and the European Community (LSHB-CT-2004-005242 CONSERT and PERSIST). C.F. is supported by an ANRS (Paris, France) postdoc fellowship.
Authorship
Contribution: C.F., F.-L.C., and E.V. designed the research and analyzed data; C.F., C.C., E.G., and E.V. performed research; C.F., F.-L.C., and E.V. wrote the paper; and D.N. and S.J.R. contributed vital new reagents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: François-Loïc Cosset, EVIR, Inserm U758. ENS de Lyon, 46 Allée d'Italie, 69364 Lyon Cedex 07, France; e-mail: flcosset@ens-lyon.fr or els.verhoeyen@ens-lyon.fr.
References
Author notes
*F.-L.C. and E.V. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal