In this issue of Blood, Lapalombella and colleagues uncover yet another consequence of exposure to the pleiotropic drug lenalidomide: the reduction of the surface receptor CD20 on leukemic B cells from CLL patients.
Lenalidomide is one of a series of immunomodulatory drugs (IMiDs) that have been tested in a variety of malignant and nonmalignant disorders. The history and application of this family of drugs dates back to the 1950s when thalidomide, a synthetic glutamic acid derivative, was initially developed as an anticonvulsant for the treatment of epilepsy. The IMiDs exhibit a multitude of biologic effects on cytokine and cell-mediated responses that likely play a role in their now well-documented activity in many human diseases. Lenalidomide is a second generation IMiD compound, developed by chemical modification of the structural backbone of thalidomide to enhance immunomodulatory potency and minimize the dose-limiting neurotoxic effects. There is appreciable clinical activity of this drug in hematologic malignancies including myelodysplastic syndrome, multiple myeloma, and chronic lymphocytic leukemia (CLL).1-4 Many potential mechanisms of action exist for lenalidomide, including inhibition of angiogenesis, enhancement of immune system function, inhibition of tumor stromal cell interactions, and blockade of actions of various cytokines.5 While these functions await clear delineation in relation to how this drug may work in hematologic malignancy, one of its putative and relevant functions could be to enhance antibody-dependent cellular cytotoxicity (ADCC). In the article by Lapalombella et al, they have studied ADCC mediated by natural killer (NK) cells toward CLL B cells in the presence of rituximab and found it to be suppressed when B cells were preincubated with lenalidomide. Rituximab is a key component of a number of CLL treatment regimens. The authors' comment that lenalidomide did not alter alemtuzumab-mediated ADCC, demonstrating that the suppression of ADCC is not global. However, it would have been helpful to have included studies on NK-cell killing function toward standard NK targets, such as K562 cells, In addition, data on whether other non-CLL CD20-expressing lymphoma cells had changes in rituximab-mediated ADCC in the presence of lenalidomide would be informative.
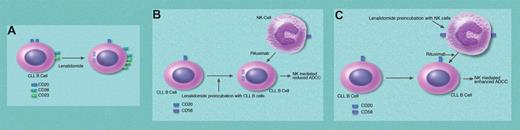
Lenalidomide can modulate levels of CLL B-cell and NK receptors and NK cell–mediated ADCC. Panel A shows the down-regulation of CD20 via internalization while increasing CD38 and CD23 on CLL B cells. Panel B shows that preincubation of CLL B cells with lenalidomide results in reduced NK-mediated ADCC in the presence of rituximab. Panel C shows that preincubation of NK cells with lenalidomide generates “activated” NK cells with increased levels of CD56 and increases in NK-mediated ADCC in the presence of rituximab. Professional illustration by Marie Dauenheimer.
Lenalidomide can modulate levels of CLL B-cell and NK receptors and NK cell–mediated ADCC. Panel A shows the down-regulation of CD20 via internalization while increasing CD38 and CD23 on CLL B cells. Panel B shows that preincubation of CLL B cells with lenalidomide results in reduced NK-mediated ADCC in the presence of rituximab. Panel C shows that preincubation of NK cells with lenalidomide generates “activated” NK cells with increased levels of CD56 and increases in NK-mediated ADCC in the presence of rituximab. Professional illustration by Marie Dauenheimer.
Other in vitro salient findings of the study included significant down-regulation of CD20 (but increases in CD23 and CD38) on primary CLL B cells exposed to lenalidomide, a reduction of rituximab-mediated apoptosis of CLL B cells by NK cells, and the realization that this latter reduction was due to internalization of CD20. All these findings, in total, suggest that if combinations of lenalidomide and rituximab are to be used in the treatment of CLL, a better strategy might be to avoid the simultaneous use of both these drugs. Indeed, the authors suggest that their preclinical work can be used to guide the design of trials using these 2 agents.
Before modifying regimens however, there are several confounding issues. First, there is no clear indication of exactly how rituximab works in vivo to generate effective clearance of CLL B cells. Thus, cells other than NK cells may be the primary in vivo effectors in rituximab-mediated ADCC. In addition, while the authors do not show this data, they comment that monocyte ADCC in the presence of lenalidomide is not affected. Secondly, the trial currently being run by Dr Asher Chanan-Khan for relapsed/refractory CLL employs first lenalidomide alone and then (at time of minimal or no response) adds rituximab to lenalidomide. The results of this trial indicate that CLL patients who have initially received lenalidomide can have enhanced clinical responses with the addition of rituximab (Chanan-Khan, verbal communication, July 2008). Until there is a clear dissection of the exact mechanisms of rituximab-mediated clearances of CLL B cells, it would seem premature to alter current strategies. However, this work does make a very strong case for further studies of monoclonal antibodies in xenograft mouse models where the timing of lenalidomide and other monoclonal antibodies targeted to CLL B cells is tested.
This study also found that lenalidomide-mediated internalization of CD20 can be used to enhance the ability to deliver oligonucleotide-based therapy by using CD20 immune liposomes. The investigators are to be congratulated for recognizing that the internalization of CD20 could be a novel approach for delivery of treatment vectors, such as microRNA or siRNA, that can target critical molecules in CLL B cells. This latter finding underscores the fact that the investigation of drugs such as lenalidomide must be continued not only to determine the exact nature of the mechanism of action in the presence or absence of other drugs/monoclonal antibodies but also in the context of specific diseases. In total, this work has shown the “yin and yang” of lenalidomide: decreases in rituximab-mediated ADCC of CLL B cells by NK cells when CLL B cells are preincubated with the drug, increases in ADCC if NK cells are first exposed to lenalidomide, and increases in CD23 and CD38 but reduced membrane levels of CD20 via internalization. Further work in CLL and other B-cell malignancies that evaluate the key mechanisms of action for lenalidomide toward leukemic/lymphoma cells are surely going to uncover critical information for the clinical trialist.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal