Abstract
B cells are well-known mediators of humoral immunity and serve as costimulators in the generation of T cell–mediated responses. In several mouse models, however, it was observed that B cells can also down-regulate immune reactions, suggesting a dual role for B cells. Due to this discrepancy and so far limited data, we directly tested the effects of primary human B cells on activated CD4+ T helper cells in vitro. We found that under optimal costimulation large, activated CD25+ B cells but not small CD25− B cells induced temporary T-cell anergy, determined by cell division arrest and down-regulation of cytokine production. In addition, large CD25+ B cells directly induced CD95-independent apoptosis in a subpopulation of activated T cells. Suppression required direct B-T-cell contact and was not transferable from T to T cell, excluding potential involvement of regulatory T cells. Moreover, inhibitory effects involved an IL-2–dependent mechanism, since decreasing concentrations of IL-2 led to a shift from inhibitory toward costimulatory effects triggered by B cells. We conclude that activated CD25+ B cells are able to costimulate or down-regulate T-cell responses, depending on activation status and environmental conditions that might also influence their pathophysiological impact.
Introduction
The adaptive immune response is a tightly regulated process involving proper initiation and termination of an immune reaction. Both tasks are performed via a network of cytokines, antigen-presenting cells (APCs), effector cells, and regulatory cell populations. Among the latter, regulatory T cells (Tregs) have been identified, which are able to down-regulate responses of effector T cells toward both foreign and self-antigen, and are mandatory for maintenance of tolerance.1
Little is known so far about other lymphocyte populations, such as B cells, in this process: B cells perform a wide array of functions facilitating the immune response, among them humoral immunity, presentation of antigen and costimulatory molecules, and production of cytokines.2,3 On the other hand, B cells can participate in the induction of immune tolerance4,5 and promotion of naive T-cell differentiation toward a Th2 phenotype.6 In autoimmune diseases, B cells have been widely ascribed a pathogenic role via production of autoreactive antibodies (Abs), enhanced priming of self-reactive T cells or blocking of regulatory T cells.7-9 However more and more hints are pointing to the existence of B-cell subsets that can down-regulate autoimmune reactions or promote recovery from chronic inflammation, suggesting a dual role of B cells in immune responses.10,11 This was first demonstrated in respective mouse models, where absence of B cells led to an exacerbated course of diseases such as autoimmune encephalomyelitis, chronic colitis, schistosoma infection, and allergy.12-15 Further research revealed different potential mechanisms, among them signaling via FcγR,13,14 down-regulation of Th1 response,16,17 production of immunosuppressive cytokines,18-21 and induction of Tregs.22,23
Unfortunately in many of these in vivo mouse models, “third-party” effects, for example, via dendritic cells could not always be excluded. Besides, genetically altered mouse strains do not easily allow translation of the obtained results into a normal background or, even further, into the human system.
Due to the discrepancy of available reports and the so far limited experimental means, we decided to set up an in vitro model with primary human B cells from peripheral blood (PB) to test their effects on TCR-activated CD4+ T-helper cells.
We found that upon appropriate stimulation, activated B cells were able to down-regulate T-cell responses and induce temporary anergy as well as apoptosis. We conclude that human B cells are able to develop regulatory and effector functions, which assigns them an essentially new role as modulators of immune responses and might influence their pathophysiological impact as well.
Methods
Antibodies, cytokines, and reagents
Antibodies used for flow cytometry are as follows: PE-anti-CD19, -CD25, -CD27, -CD38, -CD69; APC-anti-CD4, -CD19, -CD38; FITC-anti-IgD (all from BD Biosciences, San Jose, CA); FITC-anti-CD4, -CD19, -CD20, CD25, -CD38, -CD54, -CD71, -CD80, -CD86; PE-anti-CD4; PeCy5-anti-CD4, -CD19, -CD25 (all from Beckman Coulter, Hialeah, FL); PE-anti-B7-H1 and -PD-1 (eBiosciences, San Diego, CA); and FITC-anti-GITR (R&D systems, Minneapolis, MN).
Antibodies used for blocking and neutralization experiments are as follows: anti-hIL-10 (clone 23738), anti-hTGFβ1,2,3 (clone 1D11), anti-CD25 (IL-2Ra, clone 22722.2), anti-CD122 (IL-2Rβ, clone 27302), anti-CD95L (clone NOK1 and clone NOK2), anti-CD54 (clone BBIG-I1) (all from R&D systems), anti-hTNFa (clone 1825 [R&D systems], clone D2E7 [adalimumab], and clone cA2 [remicade]), anti-B7-H1 (clone MIH1; eBiosciences), and CTLA4-Ig (Impact Biotechnologies, Hamburg, Germany), each added to cultures at 10 μg/mL if not indicated otherwise.
Antibodies used for stimulation are as follows: human CD3-specific antibody OKT3 (ATCC, Manassas, VA) was used at a final concentration of 1.25 μg/mL; CD28 mAb, at 0.1 μg/mL (clone CD28.2; eBiosciences); and anti-IgM/IgG goat-α-human F(ab′)2 (Jackson ImmunoResearch Laboratories, West Grove, PA), at a final concentration of 20 μg/mL. Formalinized Cowan I strain of Staphylococcus aureus (SAC, Pansorbin; Calbiochem, San Diego, CA) was used at a final concentration of 1/10 000 (vol/vol) and purified phosphorothioate-modified CpG-oligo ODN2006 (5′-tcgtcgttttgtcgttttgtcgtt-3′; TIB Molbiol, Berlin, Germany), at a concentration of 2 μM.
Human rIL-2 and rIL-4 were obtained from Peprotech (Rocky Hill, NJ) and used at 100 U/mL, if not indicated otherwise.
Separation procedures
Peripheral blood mononuclear cells (PBMCs) from heparinized blood samples were isolated via centrifugation over Ficoll-Hypaque (LSM; density, 1.077 g/mL; PAA, Pasching, Austria). Viability was determined by trypan blue exclusion. B cells were positively selected by CD19-coated magnetic beads (Miltenyi Biotec, Bergisch-Gladbach, Germany), according to the manufacturer's instructions and were more than 98% pure, as assessed by immunofluorescence. When indicated, subfractions were sorted on a FACS-Vantage-SE cell sorter (BD Biosciences) after incubation with fluorochrome-labeled Abs. Highly purified CD4+ cells were isolated with a negative CD4+ T-cell isolation kit (Miltenyi Biotec) and were more than 97% CD4+.
Tonsils were provided by routine tonsillectomy from the clinics for Head and Neck Surgery University of Heidelberg. Briefly, fresh tonsils were minced, diluted in PBS, and filtered through a 70-μm pore size nylon filter (BD Biosciences). The cell suspension was centrifuged over Ficoll-Hypaque and further cell separations were performed as described in the preceding paragraph for PBMCs.
Cell cultures
RPMI 1640 medium (PAA) was supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin (all from Gibco-BRL, Carlsbad, CA), 1 mM sodium-pyruvate, 2 mM L glutamine, 10 mM Hepes, 0.55 mM MEM nonessential amino acids (all from PAA), and 0.05 mM 2-mercaptoethanol (Sigma-Aldrich, St Louis, MO), at 37°C and 5% CO2 in a fully humidified atmosphere.
B cells were cultured in 24-well plates at 5 × 105 B cells/mL in presence of respective stimuli. All stimuli were tested for possible effects on T-cell activation and found to be negative. For selective blocking experiments, B cells were incubated with Fc-Block (intravenous immune globulin, 1:10 [vol/vol]) diluted in phosphate-buffered saline (PBS) (PAA) for 10 minutes at 4°C, followed by 20 μg/mL of respective Abs for 25 minutes in complete media and followed by several washes in PBS. For T-cell activation, culture plates were coated with OKT3 in PBS at 4°C overnight and washed before use.
Proliferation assays
B cells were seeded at a concentration of 2.5 × 104/well with 2.5 × 104 CD4+ T cells in a final volume of 200 μL/well in OKT3-coated (cCD3) 96-well round-bottom plates with or without IL-2 in triplicates for 96 hours, unless indicated otherwise. Cells were pulsed with 0.5 μCi (.0185 MBq)/well [3H]thymidine (TdR; Hartmann Analytik, Braunschweig, Germany) for the last 16 hours of culture and harvested on an semiautomatic cell harvester (Tomtec, Hamden, CT), and [3H]TdR incorporation was quantified in a TopCount Scintillation Counter (Perkin-Elmer, Waltham, MA). B cells were irradiated at first (30 Gy; Figures 1;2), but this was suspended for further cultures exceeding 4 days, since controls showed that effects were comparable (proliferation rate of nonirradiated B cells did not exceed 3000 cpm anymore within the cocultures), while B-cell viability was retained.
Alternatively, T cells were stained with the membrane dye PKH-26 (Sigma-Aldrich), and fluorescence intensity at day 0 on a flow cytometer was set to 100%. Percentage of dividing T cells was calculated by subtraction of percentage of PKH-26+ T cells at day x from PKH-26+ T cells at day 0 (= 100%) after variable incubation times. Counterstaining with fluorochrome-labeled Abs for CD19 and CD19/CD4, respectively, was performed during each measurement.
In experiments using cell culture inserts, 1.25 × 105 B cells were cultured with equal numbers of PKH-26–stained T cells in OKT3-coated 24-well plates at a total volume of 1 mL. B cells were physically separated from T cells by placing them into cell culture inserts with a 0.4-μm semipermeable PCF-membrane (Millicell; Millipore, Billerica, MA).
Flow cytometry
Cells were stained with combinations of fluorochrome-labeled Abs according to standard procedures, including a blocking step with Fc-Block for 10 minutes at 4°C before incubation with fluorochrome-labeled Abs. Expression of respective surface markers was quantified on a double laser (488 nm and 637 nm) 5-color FACScan flow cytometer (BD Biosciences, upgraded by Cytek, Fremont, CA). Propidium iodide (PI)–positive dead cells were gated out. Cell clusters were separated by reverse pipetting, and remaining CD4+CD19+ cell doublets (3%-7%) were excluded from analysis. Sorting of different cell populations was performed on a FACS-Vantage-SE cell sorter (BD Biosciences) and viability was checked during reanalysis.
Measurement of apoptosis by flow cytometry
Fluorochrome-labeled cells were washed in annexin V labeling buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2), followed by addition of 1 μL annexin V-FITC, 0.5 μg PI, and incubation for 30 minutes at 4°C. During subsequent analysis, viable lymphocytes were gated using forward-scatter versus side-scatter characteristics, and T-cell apoptosis (in %) was determined by gating for the annexin V-FITC–positive cells within the CD4+CD19−PI− population.
Immunofluorescence microscopy
Cocultures were stained with respective surface Abs and annexin V, according to respective standard protocols, immobilized by addition of glycerol and visualized by a Olympus IX70 inverted fluorescence microscope (Olympus Optical, Hamburg, Germany) equipped with a digital image acquisition and processing system (analySIS 3.2; Soft Imaging System, Münster, Germany). Appropriate fluorescence filter sets for FITC, CY3, and APC were used for the detection of antibodies labeled with the corresponding fluorescent dye. Cells were observed with a 40× immersion objective (40× HI; Olympus Optical), connected to a video camera (Colorview XS; Soft Imaging Systems; NA of 40× objective: 1.0).
Enzyme-linked immunosorbent assay
IL-10 and IFNγ enzyme-linked immunosorbent assay (ELISA, duo set; R&D Systems) were performed from cell culture supernatants after 48-hour incubation in doublets, and cytokine concentrations were quantified according to the standard curves.
Statistical analysis
Data from individual experiments are expressed as mean plus or minus SEM. Statistical significance was determined using the paired Student t test and P value less than .05 was considered statistically relevant.
Results
Polyclonally activated B cells inhibit CD4+ T-cell proliferation
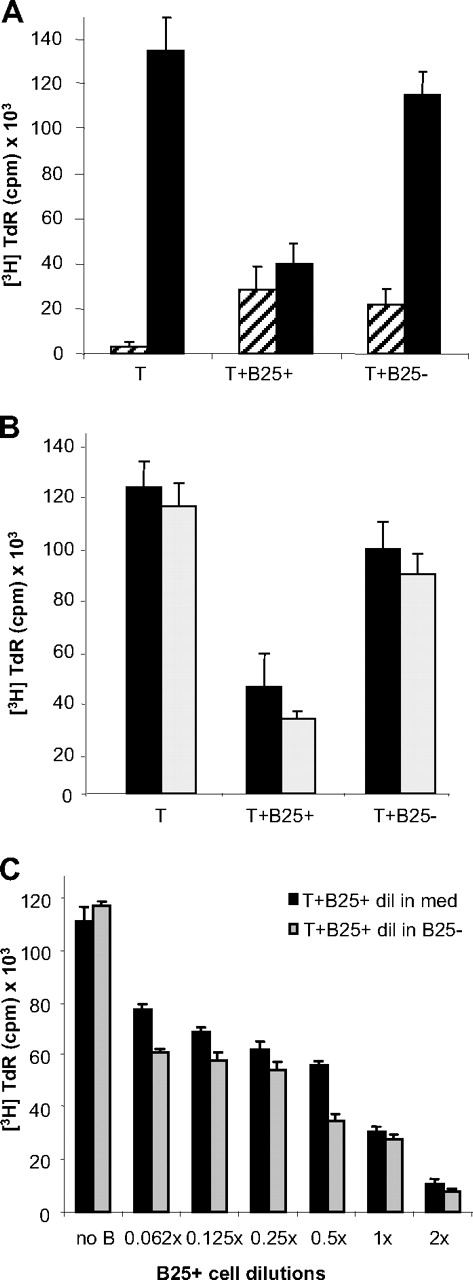
In preliminary experiments, purified human B cells from PB were activated via their BCR by anti-IgG/IgM-cross-linking (αIg) or S aureus Cowan I antigen (SAC). After 3 days, B cells were washed, irradiated, and cocultured in equal numbers with freshly isolated autologous CD4+ T cells, serving as responder cells in presence of CD3-mAb. Since the activated B-cell populations were expressing variable high numbers of IL-2R, all cocultures were provided with exogenous IL-2 to assure equal and unlimited availability of IL-2 for the T cells and optimal stimulatory conditions. After 4 days, T-cell proliferation was determined by 3H-thymidine ([3H]-TdR) incorporation (Figure 1A): Whereas unstimulated B cells had no significant effect on T-cell proliferation, αIg-stimulated B cells inhibited T-cell proliferation to about 30% and SAC-stimulated B cells to about 50%. Since SAC stimulation led to a stronger activated phenotype than αIg, according to size and CD25 (IL-2Rα) expression (SAC: 48% ± 8%, αIg: 31% ± 4%, unstimulated: 8% ± 5%,), we asked whether highly activated B cells could be the true source of inhibitory activity.
Activated B cells mediate inhibition of Th-cell proliferation. (A) Effect of differently activated B cells on CD4+ Th-cell proliferation: Equal amounts of B cells were added unstimulated, or after prestimulation for 3 days with SAC or αIg to Th cells and cCD3+ IL-2. [3H]TdR incorporation was measured after 4 days of culture. Mean plus or minus SEM of 3 representative experiments. (B) Separation of SAC-activated B cells into 2 major populations, according to cell size distribution and activation status. Shown is 1 representative sort gate for separation of small CD25− (R2 and R4 and not R6) and large CD25+ (R3 and R5 and not R6) B cells after prestimulation for 3 days
Activated B cells mediate inhibition of Th-cell proliferation. (A) Effect of differently activated B cells on CD4+ Th-cell proliferation: Equal amounts of B cells were added unstimulated, or after prestimulation for 3 days with SAC or αIg to Th cells and cCD3+ IL-2. [3H]TdR incorporation was measured after 4 days of culture. Mean plus or minus SEM of 3 representative experiments. (B) Separation of SAC-activated B cells into 2 major populations, according to cell size distribution and activation status. Shown is 1 representative sort gate for separation of small CD25− (R2 and R4 and not R6) and large CD25+ (R3 and R5 and not R6) B cells after prestimulation for 3 days
The large CD25+ but not the small CD25− B-cell population has suppressive activity
In the following experiments, B cells were activated for 3 days with SAC and IL-2 and separated by flow cytometric cell sorting into 2 major fractions according to cell size and CD25 expression (Figure 1B): large FSChi CD25+ B cells (lgB25+) and small FSClow CD25− B cells (smB25−). These different B-cell subpopulations were cocultured with responder Th cells as described. The results (Figure 2A) show that only the fraction of activated large B cells was able to suppress T-cell proliferation effectively (40 103 ± 9289 cpm vs 134 942 ± 15 159 cpm), compared with the small B cells (115 112 ± 9915 cpm), which had no biologically significant effect. Therefore enrichment of these cells showed that the suppressive effect had increased up to 72%. In contrast, stimulation with αCD3 alone led to an overall low proliferation rate and a slightly costimulatory effect by the B cells, independent from their activation status. Inhibitory effects were reproducible also with αCD28 or αCD28 + IL-2 (data not shown) and with T cells prestimulated by αCD3 + IL-2 (Figure 2B) or αCD28, suggesting that initial activation does not abolish susceptibility to the negative regulatory effects exerted by the B cell.
The large CD25+ B cells mediate suppression of activated Th cells. (A) Proliferation assay of cCD3-stimulated Th cells alone or in presence of lgB25+ or smB25− cells after 4 days in presence (■) or absence (▨) of IL-2. Bars represent mean of 9 independent experiments plus or minus SEM. (B) Proliferation of prestimulated ( ) or freshly isolated (■) Th cells in presence of lgB25+ or smB25−B cells after 4-day culture with IL-2. Mean plus or minus SEM of 3 representative experiments. (C) Proliferation of Th cells in presence of different concentrations of lgB25+ cells, diluted in cell culture media (■) or in B25− cells (
) or freshly isolated (■) Th cells in presence of lgB25+ or smB25−B cells after 4-day culture with IL-2. Mean plus or minus SEM of 3 representative experiments. (C) Proliferation of Th cells in presence of different concentrations of lgB25+ cells, diluted in cell culture media (■) or in B25− cells ( ). Data expressed as mean plus or minus SEM of triplicates from one representative experiment.
). Data expressed as mean plus or minus SEM of triplicates from one representative experiment.
The large CD25+ B cells mediate suppression of activated Th cells. (A) Proliferation assay of cCD3-stimulated Th cells alone or in presence of lgB25+ or smB25− cells after 4 days in presence (■) or absence (▨) of IL-2. Bars represent mean of 9 independent experiments plus or minus SEM. (B) Proliferation of prestimulated ( ) or freshly isolated (■) Th cells in presence of lgB25+ or smB25−B cells after 4-day culture with IL-2. Mean plus or minus SEM of 3 representative experiments. (C) Proliferation of Th cells in presence of different concentrations of lgB25+ cells, diluted in cell culture media (■) or in B25− cells (
) or freshly isolated (■) Th cells in presence of lgB25+ or smB25−B cells after 4-day culture with IL-2. Mean plus or minus SEM of 3 representative experiments. (C) Proliferation of Th cells in presence of different concentrations of lgB25+ cells, diluted in cell culture media (■) or in B25− cells ( ). Data expressed as mean plus or minus SEM of triplicates from one representative experiment.
). Data expressed as mean plus or minus SEM of triplicates from one representative experiment.
To determine the amount of B cells necessary for suppression, we created different ratios of activated B cells to T cells by mixing lgB25+ cells with smB25− cells in increasing dilutions and by keeping a constant number of 1:1 B/T ratio. Alternatively, lgB25+ cells were diluted in cell culture media (Figure 2C). The results showed clearly that even when the total B-cell number remained constant, the extent of suppression was dependent on the amount of the lgB25+ cells within the population. Remarkably, addition of lgB25+ cells in a 2:1 ratio to the T cells further increased inhibition up to 90%. In experiments with sorted CD25+ αIg-stimulated B cells, a similar dependency was found, though the effects were still not as striking (maximum 50% inhibition, data not shown), indicating that—apart from CD25+—differences in phenotype and function of B-cell populations might influence regulatory capabilities. This hypothesis was also supported by the fact that CD25+ B cells, which comprise about 5% to 15% of the total B cells, sorted directly out of PB, had no significant impact in our system. In addition, sorting of SAC-activated B cells for CD25high, as successfully performed for enrichment of human natural regulatory T cells,24 did not increase inhibition significantly more (data not shown).
Interestingly, also prestimulation of B cells by use of CpG-oligos (ODN2006) led to T-cell suppression comparable with SAC (Table 1): Here again, only large activated B cells suppressed to about 70% in a 1:1 ratio (30 358 ± 10 048 cpm vs 99 449 ± 11 593 cpm; n = 3) and about 84% in a 2:1 ratio (16 323 ± 7824 cpm), indicating that also bacterial DNA (containing unmethylated CpG motifs) can lead to B-cell–mediated T-cell suppression as soon as it leads to sufficient B-cell activation.
Inhibitory properties of CpG-activated B cells
| Ratio B cell/T cell . | % T-cell inhibition . | |
|---|---|---|
| B large . | B small . | |
| 0.062:1 | 17 ± 4 | 6 ± 4 |
| 0.125:1 | 22 ± 3 | 9 ± 5 |
| 0.25:1 | 36 ± 3 | 15 ± 4 |
| 0.5:1 | 41 ± 2 | 17 ± 1 |
| 1:1 | 69 ± 9 | 20 ± 3 |
| 2:1 | 84 ± 7 | 31 ± 7 |
| Ratio B cell/T cell . | % T-cell inhibition . | |
|---|---|---|
| B large . | B small . | |
| 0.062:1 | 17 ± 4 | 6 ± 4 |
| 0.125:1 | 22 ± 3 | 9 ± 5 |
| 0.25:1 | 36 ± 3 | 15 ± 4 |
| 0.5:1 | 41 ± 2 | 17 ± 1 |
| 1:1 | 69 ± 9 | 20 ± 3 |
| 2:1 | 84 ± 7 | 31 ± 7 |
Data represent mean inhibition of T-cell proliferation ± SD from 3 donors after 4-day culture with CD3/IL-2 and autologous B cells, prestimulated with CpG and IL-2 for 3 days and FACS-sorted according to cell size. Inhibition was calculated as proliferation of T cells in presence of B cells in relation to proliferation of T cells alone.
Inhibitory effects involve long-lasting cell division arrest
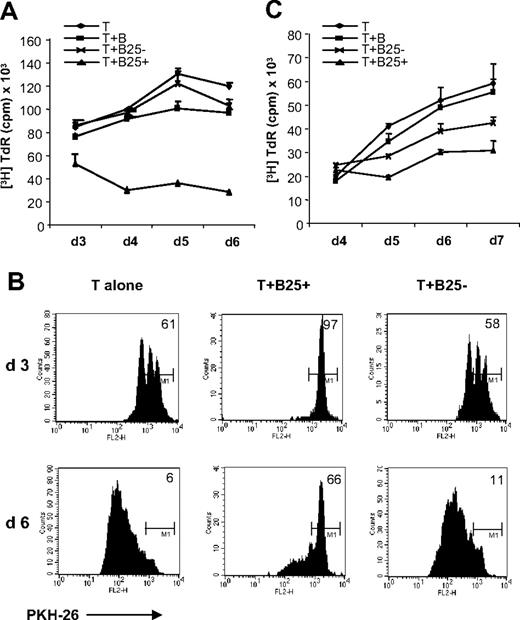
Measurement of [3H]-TdR incorporation at different time points revealed that inhibitory activity increased over time: Although inhibition started with 38% at day 3, it increased up to 77% at day 6 of the cocultures (Figure 3A). Additional staining of T cells with PKH-26 at day 0 of the culture revealed that the majority of T cells remained under cell division arrest (Figure 3B). Long-term suppression was also confirmed in an MLR, where autologous B and T cells were mixed with allogeneic irradiated PBMCs (Figure 3C) for more than 7 days, confirming that our observations were not solely based on the CD3 antibody stimulus but could also be reproduced in the context of recognition of foreign MHC II. The slightly reduced suppression (50%) could be caused by the PBMCs in the culture that might impede close B-T-cell contact. Interestingly, a slight suppression by the initially smB25− population could be observed over time. Since smB25− cells started to up-regulate CD25 in the cocultures, it is tempting to speculate that some of these might have acquired regulatory functions as well, perhaps via another mechanism. Although no αCD3 was added to the MLR cultures, exogenous IL-2 had to be present for suppressive activity. This was consistent with CD3 stimulation and led us to wonder about the requirements for suppressor activity.
lgB25+ cells induce long lasting T-cell division arrest. (A) T cells were stimulated alone (rhombus), with unstimulated B cells (square), smB25− cells (cross), and lgB25+ cells (triangle) as usual in presence of cCD3 and IL-2. Data are expressed as mean plus or minus SEM of triplicates from 1 representative experiment of at least 3. (B) T-cell division after 3 and 6 days, measured by PKH-26 dilution. M1 relates to 100% PKH-26–positive cells at day 0. (C) MLR with autologous B and T cells (each 25 000) and allogeneic irradiated PBMCs (50 000) in presence of IL-2. Data are expressed as mean plus or minus SEM of triplicates from 1 representative experiment of at least 3 (symbols as in panel A)
lgB25+ cells induce long lasting T-cell division arrest. (A) T cells were stimulated alone (rhombus), with unstimulated B cells (square), smB25− cells (cross), and lgB25+ cells (triangle) as usual in presence of cCD3 and IL-2. Data are expressed as mean plus or minus SEM of triplicates from 1 representative experiment of at least 3. (B) T-cell division after 3 and 6 days, measured by PKH-26 dilution. M1 relates to 100% PKH-26–positive cells at day 0. (C) MLR with autologous B and T cells (each 25 000) and allogeneic irradiated PBMCs (50 000) in presence of IL-2. Data are expressed as mean plus or minus SEM of triplicates from 1 representative experiment of at least 3 (symbols as in panel A)
B cell–mediated suppression is critically dependent on IL-2
To investigate the correlation between activation signals and suppression, we tested different concentrations of IL-2 (Figure 4A). Under influence of low IL-2 concentrations (up to 10 U/mL), lgB25+ cells had a slightly costimulatory effect on T-cell proliferation. Concentrations from IL-2 higher than 50 U/mL led to a striking increase of DNA replication among T cells cultured alone or with smB25− cells, whereas presence of lgB25+ cells kept T-cell proliferation below 30%, as observed before. PKH-26 labeling moreover revealed that initial percentages of dividing T cells in presence of lgB25+ cells decreased with increasing IL-2 concentrations (Figure 4B). These data suggest that inhibition of T cells correlates directly with the amount of IL-2 offered and cannot result from competition for IL-2 with the B cells, as sometimes claimed in context with Treg function.25
B cell–mediated Th-cell suppression is critically dependent on IL-2. (A-C) Th cells were incubated alone (■), with lgB25+ (▨) or smB25− (▤) cells in presence of cCD3 and different concentrations of IL-2 or without IL-2 (med). (A) [3H]TdR incorporation of T cells. Data are expressed as mean plus or minus SEM of triplicates from 1 representative experiment. (B) PKH-26 assay at day 6 (mean ± SEM of 3 independent experiments). (C) Blockade of IL-2Rαβ by addition of Abs directly to culture or by preincubation of lgB25+ cells (B*) alone; cultures provided with cCD3 and IL-2 (100 U/mL). Mean plus or minus SEM and P value of 5 independent experiments.
B cell–mediated Th-cell suppression is critically dependent on IL-2. (A-C) Th cells were incubated alone (■), with lgB25+ (▨) or smB25− (▤) cells in presence of cCD3 and different concentrations of IL-2 or without IL-2 (med). (A) [3H]TdR incorporation of T cells. Data are expressed as mean plus or minus SEM of triplicates from 1 representative experiment. (B) PKH-26 assay at day 6 (mean ± SEM of 3 independent experiments). (C) Blockade of IL-2Rαβ by addition of Abs directly to culture or by preincubation of lgB25+ cells (B*) alone; cultures provided with cCD3 and IL-2 (100 U/mL). Mean plus or minus SEM and P value of 5 independent experiments.
To test whether IL-2 might rather serve the suppressive capabilities of the B cell or susceptibility to inhibition by the T cell, a selective preincubation of B cells with blocking Abs against CD25 and CD122 subunits of IL-2R was performed. With viability of the B cells retained, T-cell proliferation was partly reconstituted by about 50% (from 22% back to 46%, Figure 4C). It is conceivable that preblocking with Ab is not sufficient to permanently block signaling via IL-2 receptors, up-regulated on the B cells later on. However the limited availability of IL-2 apparently had alleviated suppressive effects, whereas high concentrations (or fully available receptors) rather promoted it, altogether assigning a special role to this cytokine in generation of inhibitory B cells.
Interestingly addition of the important B-cell survival factor and γ chain cytokine IL-4 instead of IL-2 did not lead to observed cell division arrest and, applied together with IL-2, it did not interfere with inhibition—neither did addition of neutralizing Abs against immunosuppressive IL-10 or TGFβ. Besides, IL-10 was greatly diminished in the supernatants of B-T cocultures in parallel with IFN γ, and B cell–specific production was negligible (data not shown).
Suppression is cell contact dependent and requires constant presence of SAC-activated B cells
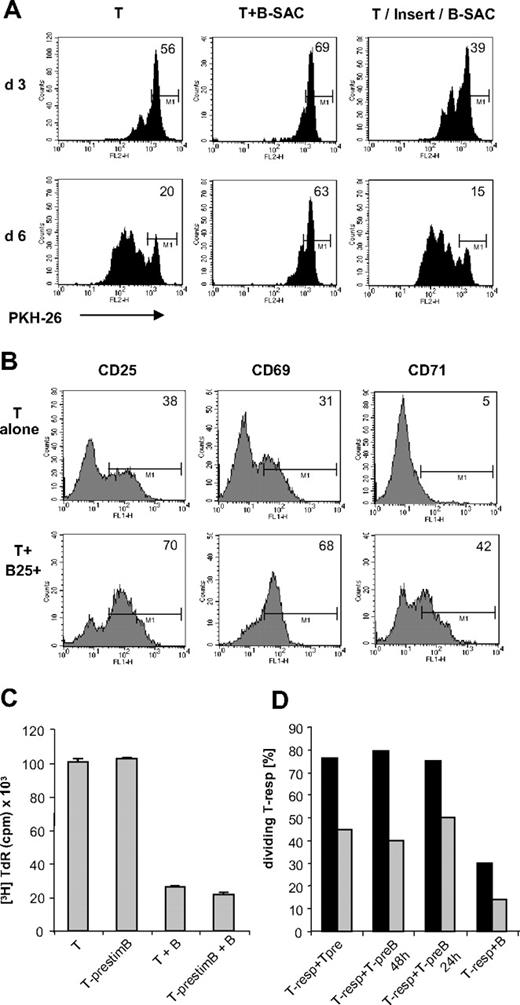
Since supernatants from SAC-stimulated B cells alone or from B-T cocultures had no inhibitory effect on the T cells (data not shown), we tested whether the suppressive effects are dependent on cell contact: SAC-stimulated B cells were cocultured with PKH-26–stained T cells or separated by semipermeable cell culture inserts, under presence of αCD3/IL-2. Although in T-B coculture the majority of T cells remained suppressed as expected (Figure 5A), separation from B cells did not affect T-cell proliferation, but rather supported it at first, suggesting that direct cell contact is a prerequisite for regulatory activity and could potentially outbalance soluble costimulatory factors. In addition, keeping the cocultures in flat-bottom plates instead of round-bottom plates reduced the inhibitory effects, though did not abrogate them, probably due to lesser cell contact at the cell concentrations used (60 000 cells).
T-cell inhibition requires cell contact and constant presence of activated B cells. (A) PKH assay of Th cells stimulated alone or together with SAC-activated B cells, or separated by cell culture insert (T/Insert/B-SAC) after 3 and 6 days. (B) Surface expression of CD71, CD69, and CD25 on gated CD4+ T cells after 24-hour stimulation with cCD3/IL-2 in presence or absence of lgB25+ cells. (C) T cells prestimulated alone (T-prestim) or with lgB25+ cells (T-prestimB) for 48 hours and restimulated with IL-2 or IL-2+ lgB25+ cells. Data are expressed as mean plus or minus SEM of triplicates from 1 representative experiment. (D) Coculture of PKH-26+ responder T cells (Tresps) with Tprestim or with TprestimB for 24 or 48 hours. Tresps were used fresh ( ) or prestimulated for 48 hours (■). PKH-26 was measured after 3 days.
) or prestimulated for 48 hours (■). PKH-26 was measured after 3 days.
T-cell inhibition requires cell contact and constant presence of activated B cells. (A) PKH assay of Th cells stimulated alone or together with SAC-activated B cells, or separated by cell culture insert (T/Insert/B-SAC) after 3 and 6 days. (B) Surface expression of CD71, CD69, and CD25 on gated CD4+ T cells after 24-hour stimulation with cCD3/IL-2 in presence or absence of lgB25+ cells. (C) T cells prestimulated alone (T-prestim) or with lgB25+ cells (T-prestimB) for 48 hours and restimulated with IL-2 or IL-2+ lgB25+ cells. Data are expressed as mean plus or minus SEM of triplicates from 1 representative experiment. (D) Coculture of PKH-26+ responder T cells (Tresps) with Tprestim or with TprestimB for 24 or 48 hours. Tresps were used fresh ( ) or prestimulated for 48 hours (■). PKH-26 was measured after 3 days.
) or prestimulated for 48 hours (■). PKH-26 was measured after 3 days.
Interestingly, the expression of early activation markers on the T cell was not inhibited in presence of lgB25+ cells, as the T-cell population expressed homogenously CD25, CD69, and CD71 within 24 hours (Figure 5B). To determine whether these T cells could recover from suppression and continue cell cycling, we preincubated CD4+ T cells with αCD3/IL-2 with or without lgB25+ B cells as usual. After 48 hours (Figure 5C), CD19+ B cells and dead cells were removed by fluorescence-activated cell sorting (FACS) sorting, and the T cells were set into culture again in parallel with their counterparts that had been precultured without B cells. The proliferation assay after 4 days showed that the “B-primed” T cells achieved a growth rate comparable with their “unprimed” counterparts, suggesting that inhibition was reversible. In addition, these lgB25+-“primed” T cells were not able to transfer anergizing effects to PKH-26+ T responders (Figure 5D), suggesting that constant presence of the B cell is mandatory for suppression. It also confirms that the B cells do not mediate suppressive effects indirectly via regulatory T-cell populations, such as natural or induced Tregs. This was further supported by the observation that the extent of inhibition was still the same, when all CD25+ T cells containing natural Tregs were depleted by cell sorting before coculture (data not shown).
In an attempt to define the mechanism by which this kind of reversible anergy was maintained, we examined the expression of costimulatory molecules on the B cell since lack of these is known to render T cells susceptible to anergy induction.26,27 We found that the lgB25+ cells were fully capable of costimulation, as confirmed by their high expression of markers such as CD80, CD86, and CD54 (Table 2). However markers with known inhibitory activity were also found, such as B7-H1.28 Blocking of important interaction pathways between B and T cells by using blocking Abs against B-7H1, CD54, or surface TGFβ or by using CTLA-4-Ig revealed that none of them was able to effectively restore T-cell proliferation (data not shown).
Phenotypical analysis of SAC-activated B cells after 3-day culture
| % . | CD25 . | CD69 . | CD80 . | CD86 . | CD54 . | B7-H1 . | CD95 . | CD38 . | CD27 . |
|---|---|---|---|---|---|---|---|---|---|
| B large | 91 ± 6 | 33 ± 9 | 82 ± 7 | 85 ± 5 | 71 ± 8 | 45 ± 11 | 61 ± 15 | 74 ± 7 | 29 ± 6 |
| B small | 15 ± 4 | 3 ± 2 | 22 ± 6 | 22 ± 9 | 17 ± 5 | 23 ± 6 | 12 ± 4 | 13 ± 4 | 18 ± 6 |
| % . | CD25 . | CD69 . | CD80 . | CD86 . | CD54 . | B7-H1 . | CD95 . | CD38 . | CD27 . |
|---|---|---|---|---|---|---|---|---|---|
| B large | 91 ± 6 | 33 ± 9 | 82 ± 7 | 85 ± 5 | 71 ± 8 | 45 ± 11 | 61 ± 15 | 74 ± 7 | 29 ± 6 |
| B small | 15 ± 4 | 3 ± 2 | 22 ± 6 | 22 ± 9 | 17 ± 5 | 23 ± 6 | 12 ± 4 | 13 ± 4 | 18 ± 6 |
Data represent mean plus or minus SD from purified B cells of 3 donors after 3-day culture with SAC and IL-2. PI+ cells were excluded and for analysis 2 gates were set according to cell size.
Suppression involves both inhibition of cell division and induction of apoptosis
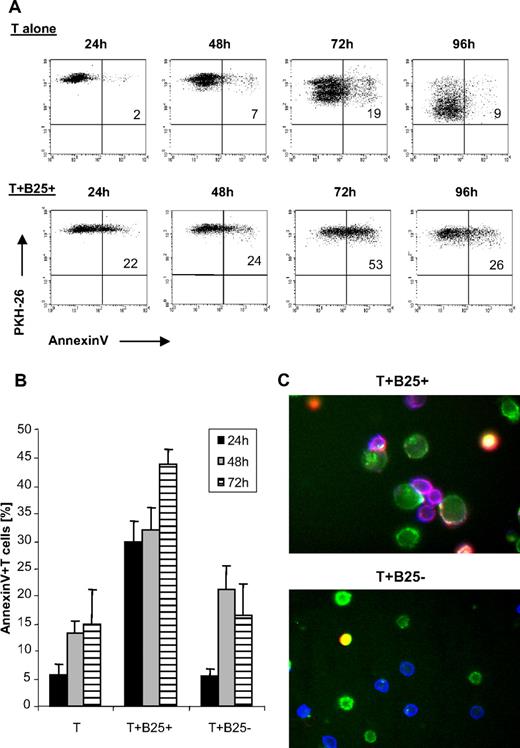
To better characterize the mechanism of suppression, we asked whether cell death played a role in our system. PKH-26–stained responder T cells were added to the lgB25+ cells and apoptosis was determined by annexin V staining versus PI. As observed before, 80% and more of the T cells stimulated alone underwent 3 and more cell divisions within 4 days, whereas in the cocultures with lgB25+ cells T cells were undergoing 1 cell division, at the most (Figure 6A). Instead, a remarkable proportion of suppressed T cells underwent apoptosis: more than 20% within the first 24 hours of coculture and more than 50% after 72 hours of culture. Cell death started almost immediately, as an increase in annexin V+ T cells was measurable already within 4 hours of incubation with lgB25+ cells (data not shown). In contrast, apoptosis of T cells alone or with smB25− cells rarely exceeded 10% to 20% within 3 days (Figure 6B). Remarkably, immunofluorescence microscopy showed many of those apoptotic T cells located within clusters, preferentially formed between T cells and lgB25+ cells, confirming action via direct B-T contact (Figure 6C).
B cell–mediated Th-cell suppression involves induction of T-cell apoptosis. (A) Annexin binding of PKH-26–labeled Th cells cultured alone or with lgB25+ cells + cCD3/IL-2 after different incubation times between 24 and 96 hours, 1 representative experiment of 4 independent experiments shown. (B) Annexin staining of T cells cultured alone or with lgB25+ or smB25− cells at different time points. Mean plus or minus SEM of 6 independent experiments shown. (C) Immunofluorescence microscopy after 24-hour incubation time; 40× magnification. Sixteen adjacent areas in a 4 × 4 array were imaged and stitched together. Images were acquired with respective filters for CD4-APC, CD19-FITC, and annexin-PE; overlay colors: green indicates B cells; blue, T cells; purple, Ax-positive T cells; and yellow, Ax-positive B cells. One representative area with T+ lgB25+ and T+ smB25− is shown.
B cell–mediated Th-cell suppression involves induction of T-cell apoptosis. (A) Annexin binding of PKH-26–labeled Th cells cultured alone or with lgB25+ cells + cCD3/IL-2 after different incubation times between 24 and 96 hours, 1 representative experiment of 4 independent experiments shown. (B) Annexin staining of T cells cultured alone or with lgB25+ or smB25− cells at different time points. Mean plus or minus SEM of 6 independent experiments shown. (C) Immunofluorescence microscopy after 24-hour incubation time; 40× magnification. Sixteen adjacent areas in a 4 × 4 array were imaged and stitched together. Images were acquired with respective filters for CD4-APC, CD19-FITC, and annexin-PE; overlay colors: green indicates B cells; blue, T cells; purple, Ax-positive T cells; and yellow, Ax-positive B cells. One representative area with T+ lgB25+ and T+ smB25− is shown.
There was also a correlation between IL-2 concentration and the apoptosis rate in all 3 groups, with differences between T cells alone and T cells plus lgB25+ cells most prominent at doses higher than 50 U/mL. Low apoptosis rates in absence of αCD3 revealed that stimulation via the TCR was indispensable for T-cell susceptibility to B cell–mediated apoptosis (data not shown).
Due to the known correlation between T-cell activation and susceptibility to apoptosis, we wondered whether activation-induced cell death (AICD) could play a role in our system. We performed blocking experiments with selective αFasL Abs, successfully proven for blocking the CD95-CD95L interaction (clones NOK-1 and NOK-2) by prelabeling of the B cells or by addition of excessive amounts into the coculture. The αFasL Abs, however, given each alone or in combination did not abolish apoptosis, and neither did αTNFα Abs or the pan-caspase inhibitor z-vad, suggesting that other mechanisms were involved (data not shown).
B cells with inhibitory capabilities can be identified in vivo
Finally, we wanted to test whether inhibitory B cells can also be found at the site of antigen response in vivo. We therefore repeated the suppressor assays with B-cell fractions isolated from human palatine tonsils, gained by routine tonsillectomy. Whereas suppressor assays were set up as before with autologous T cells and CD3/IL-2 stimulation, B cells were left unstimulated, assuming they had previous antigen contact. From the different tonsillar populations sorted according to expression of CD38 and IgD29 (Figure 7B), we found that tonsillar IgD+ CD38++ B cells—a majority of them representing the large B-cell population (Figure 7A)—reduced proliferation of autologous T cells to a level below 50% of control (43% ± 17%, n = 3). To a lesser degree, IgD+ CD38+ B cells had also some impact (65% ± 15%, n = 3; Figure 7C). Other fractions, such as CD38++ IgD− centroblasts/centrocytes revealed heterogeneous results, probably at least partly due to different amounts of further B-cell subpopulations within and different requirements on their environment: Centrocytes, for example, have been shown to quickly differentiate into memory B cells upon T-cell contact and centroblasts have been demonstrated to be sensitive to apoptosis.30,31
Activated B cells with inhibitory properties can be found in human tonsils. (A) Representative example for phenotype of purified tonsillar B cells according to CD38 and IgD expression. Upper quadrant separates CD38+ from CD38++ B cells. Large cells gated on R5; small cells gated on R4; PI+ B cells (R2) gated out. (B) Sort gates for separation of IgD+ CD38++ B cells (R6 and R1 and not R2) and IgD+ CD38+ B cells (R7 and R1 and not R2); R1 and R2 refer to the gates of panel A. (C) Suppressor assay with freshly sorted autologous cell populations and CD3 alone or CD3+ IL-2. ■ indicate T cells alone;  , T+ B-IgD+ CD38++; and ▨, T+ B-IgD+ CD38+. Data represent mean plus or minus SEM of tonsils from 3 different donors.
, T+ B-IgD+ CD38++; and ▨, T+ B-IgD+ CD38+. Data represent mean plus or minus SEM of tonsils from 3 different donors.
Activated B cells with inhibitory properties can be found in human tonsils. (A) Representative example for phenotype of purified tonsillar B cells according to CD38 and IgD expression. Upper quadrant separates CD38+ from CD38++ B cells. Large cells gated on R5; small cells gated on R4; PI+ B cells (R2) gated out. (B) Sort gates for separation of IgD+ CD38++ B cells (R6 and R1 and not R2) and IgD+ CD38+ B cells (R7 and R1 and not R2); R1 and R2 refer to the gates of panel A. (C) Suppressor assay with freshly sorted autologous cell populations and CD3 alone or CD3+ IL-2. ■ indicate T cells alone;  , T+ B-IgD+ CD38++; and ▨, T+ B-IgD+ CD38+. Data represent mean plus or minus SEM of tonsils from 3 different donors.
, T+ B-IgD+ CD38++; and ▨, T+ B-IgD+ CD38+. Data represent mean plus or minus SEM of tonsils from 3 different donors.
CD38++ IgD+ B cells have been reported to populate preferentially the dark zone of the GC, to express the proliferation marker Ki67, and to carry unmutated genes32 (according to B mature classification: Bm2-Bm3δ4δ). More work has to be done to further characterize the physiological counterpart of our in vitro–generated inhibitory B cells, but those cells might indeed most resemble the SAC-stimulated, initially naive IgD+ B cells from peripheral blood that we had used in our experiments.
Discussion
In the current study, we have shown for the first time that activated, primary human B cells from peripheral blood can regulate T helper cell responses via a new mechanism involving IL-2 signaling and direct cell contact: In presence of IL-2, polyclonally activated large CD25+ B cells down-regulated T-cell proliferation and induced apoptosis, whereas the smaller CD25− B-cell population had no significant effects, resembling freshly isolated B cells from peripheral blood in phenotype and function.
A regulatory effect of B cells was already suspected in earlier reports. However, many of those studies used unstimulated B cells or B cells after contact with soluble antigen. Those B cells were effective in Ag presentation via MHC II but weak in providing costimulatory help, leading to the well-described induction of T-cell tolerance.4,26 In our system, B cells first underwent BCR cross-linking, then expressed a full set of costimulatory markers, and formed strong clusters with T cells, followed by T-cell anergy and apoptosis, confirming that observed effects were based on a different mechanism.
A few studies have been performed, by use of immunized mice or stimulated B cells that imply the existence of different inhibitory B-cell subsets depending on mode of activation: One report describes inactivation of effector T cells and prevention of β-cell destruction in autoimmune diabetes after treatment with LPS-stimulated B cells.17 Of note, in a B cell–deficient model for this disease, B cells had first been identified to be essential for initiation of the autoimmune reaction.7 LPS-stimulated murine B cells have also been described in vitro to anergize CD8+ T cells, probably via TGFβ.21 In several autoimmune disease models, such as inflammatory bowel disease, experimental autoimmune encephalomyelitis, or collagen-induced arthritis, IL-10–producing B cells appear to play a protective role,18-20 probably via a CD40-dependent mechanism. B-T-cell contact was also necessary in our system, and could take place in form of a reciprocal dialogue. This would contribute to survival of the B cell and help to reduce bystander effects in a physiological context. Contrary to previously cited reports, however, we did not identify IL-10 or TGFβ, but did identify IL-2, to be of crucial importance for regulatory effects. Under low doses of IL-2, costimulatory effects were prominent, whereas under increasing doses inhibitory effects became obvious. This stands in contrast to Tregs, where—although certain amounts of IL-2 are necessary for maintenance—increasing IL-2 concentrations cause their inactivation.25 Based on our data, including selective blocking of IL-2R on the B cell, we suggest that high concentrations of IL-2 might sensitize the lgB25+ cell to its suppressive function and cause a switch from costimulatory to inhibitory signals. Since the CD25+ B cells directly isolated from PB—a majority of them CD27+ memory B cells (see also Amu et al33 and T.T., R.K.C.V., V.E., R.S., S.S., A.D.H., unpublished observations, 2008)—did not suppress, whereas the less mature, predominantly CD27-naive B cells from PB did, we suggest that maturation status of the B cell represents another critical parameter in this process. This hypothesis is also supported by an observation during our experiments with B-cell stimulatory CpG-rich oligonucleotides (CpG-ODNs): Although after treatment again only the large B-cell fraction (representing up to 40% of the total B-cell population) exhibited a strong inhibitory effect toward CD4+ T cells, comparable with SAC, the great majority of the B cells (> 80%) expressed CD25 on the cell surface (T.T., R.C.K.V., V.E., R.S., S.S., A.D.H., and H.-M.L., unpublished observations, 2008).
Stimulation with CpG-ODNs via TLR9 represents another pathway for T cell–independent B-cell activation and can act alone or synergistically with BCR signaling.34 Similar to BCR cross-linking, CpG-ODNs activate downstream signals though MAP kinases, resulting in NF-κB activation and expression of costimulatory molecules, production of cytokines, and antibody secretion. Of note, although CpG-ODNs induce immune activation, reports describing inhibitory effects have been published previously: Systemic and repeated treatment with CpG-ODNs led to immunosuppression, including destruction of lymphoid follicles,35 and specifically abrogation of T-cell expansion in the spleen of immunized mice.36 Taken together, this indicates an additional potential role for CpG-ODNs in downmodulation of immune responses in vivo. However, TLR9 is expressed by various cell types, including dendritic cells and macrophages. Therefore, respective in vivo models could not provide direct evidence for an involvement of B cells in this process, yet.
Future experiments will also show whether rather naive B cells, reacting to T-independent bacterial antigen or bacterial DNA (CpG-ODNs), might be most susceptible to convert into regulatory B cells (Bregs). This hypothesis would be supported by the fact that our experiments with tonsillar B cells identified a population with inhibitory properties, which interestingly belonged to an earlier maturation stage (IgD+ and CD38++ IgD+, Bm2-Bm3δ4δ) than the IgD− germinal center centrocytes and centroblasts that have already had contact with the T cell and undergo somatic mutation and class switching.31 The tonsillar CD38++ IgD+ B cells have been reported not to have completed class switching, but to secrete IgM, and to generate “atypical” germinal centers as reactions on bacterial carbohydrate Ag, which are likely involved in pathogenesis of chronic tonsillitis (TI2 antigens).32
In this context, it appears interesting that some autoimmune diseases seem to be accompanied by defects in B-cell homeostasis, for example by overrepresentation of a more mature memory phenotype or by a specific lack of naive B cells, for example, as described in rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE).37,38
It is plausible to suggest that presence of IL-2 might also contribute to sensitivity of the T cell to inhibitory effects, as is already known from AICD, by shifting them to a highly activated state.39 In our system, expression of the IL-2Rα on T cells was not interrupted and even increased in presence of lgB25+ cells and so were other early activation markers. This phenomenon has been observed with certain forms of anergy before40,41 and can be accompanied by a few cell cycles. In our system, the majority of T cells did not start any cell division at all, speaking for an immediate action in context with TCR signaling, which could help to explain why receptors such as CTLA-4 or PD-1, which take time to be up-regulated, were not involved in this process. The observed anergy was reversible, not transferable, and not accompanied by induction of Tregs. Noteworthy in the “Breg” mouse models mentioned, where activated B cells were used, an induction of Tregs was not described either and T-cell transfer experiments were not successful, whereas adoptive Breg transfer was,17,42 as evidenced by the amount of T-cell apoptosis afterward. Activated B cells have been reported to up-regulate CD95L43 and its expression has been correlated, though not fully evidenced, to B cell–mediated T-cell apoptosis in at least 2 models.17,42,44 Apoptosis, and specifically AICD, is widely accepted to be required for maintenance of self-tolerance45,46 and plays a critical role during a variety of physiological conditions and diseases from sepsis-induced lymphopenia47 to systemic autoimmune disorders.48 However, although the lgB25+ cells in our system obviously developed cytotoxic abilities toward the T cells, we did not find an involvement of the CD95 pathway. Recently apoptosis was also demonstrated in context with immune regulation by Tregs.49,50 Here, cytokine deprivation within the responder T-cell population due to consumption of γ-chain cytokines by Tregs was revealed to not only suppress their proliferation but also to induce apoptosis. However since apoptosis was detectable in our system already within few hours of coculture with inhibitory B cells under presence of sufficient amounts of exogenous IL-2, this possibility can be excluded. Another difference to the cited Treg report was also that the apoptosis pathway induced in our system was not sensitive to the pan-caspase inhibitor z-vad. However, alternative apoptosis pathways have been described before51-53 and should be considered for future research. This might also help in clarifying how the decision is made between anergy and apoptosis in the T cell.
According to our hypothesis, in a physiological environment, such as the secondary lymphoid organs, the activated B cell would be able to down-regulate T-cell responses as soon as a certain level of effector activity is reached, determined by accumulation of a critical parameter such as IL-2. This would define B cells as an additional tool for IL-2 in maintaining peripheral T-cell tolerance,1,54 next to the expansion of Tregs. The suppressor B cells would be helpful especially after confrontation with bacterial antigen, which in extreme cases can lead to sepsis or—after defective removal of effector T cells—to abnormal outgrowth of clones with accidentally self-reactive potential, as suggested for pathogenesis of several systemic autoimmune diseases. Induction of T-cell apoptosis would prevent survival of such T cells or T cells with potentially dangerous mutations, which could even lead to cell-autonomous growth. Temporary anergy on the other hand would guarantee to preserve the memory T-cell pool and would be fully reversible as soon as the B cells leave the lymph node or as the IL-2 concentration is decreasing. Which characteristics determine T-cell fate toward apoptosis or anergy we were not able to define, yet. However, we could define activation and composition of the microenvironment as crucial factors for the conversion of the B cell into a Breg, and this might help to explain the discrepant results about B-cell function gained in various experimental settings before. Overall, our findings provide evidence for a new function of human B cells during an immune response. Depending on the individual disease, effects of inhibitory B cells might appear rather desirable or detrimental to the physician. Further research in this area could put new perspectives on current treatments and offer alternatives for development of future immunotherapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kerstin Woerner for excellent flow cytometric cell sorting and Ziya Kaya for fruitful discussions and critical reading of this paper.
Authorship
Contribution: T.T., R.K.C.V., V.E., and R.S. performed the laboratory research, and analyzed and interpreted the data; V.E., R.S., S.S., and A.D.H. contributed analytical tools; and T.T. and H.L. designed the research and drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Theresa Tretter, Division of Rheumatology, Department of Medicine V, University of Heidelberg, 69120 Heidelberg, Germany; e-mail: theresa.tretter@med.uni-heidelberg.de.

![Figure 1. Activated B cells mediate inhibition of Th-cell proliferation. (A) Effect of differently activated B cells on CD4+ Th-cell proliferation: Equal amounts of B cells were added unstimulated, or after prestimulation for 3 days with SAC or αIg to Th cells and cCD3+ IL-2. [3H]TdR incorporation was measured after 4 days of culture. Mean plus or minus SEM of 3 representative experiments. (B) Separation of SAC-activated B cells into 2 major populations, according to cell size distribution and activation status. Shown is 1 representative sort gate for separation of small CD25− (R2 and R4 and not R6) and large CD25+ (R3 and R5 and not R6) B cells after prestimulation for 3 days](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-02-140087/7/m_zh80240827520001.jpeg?Expires=1767723250&Signature=cTqCckHcU2p8XLBEfiYLw~llT4P-o~eeu53yE8iUUjfNNQlA~A7m19y3LEQrQjhbSQfIhRHhB5IsAqTKe~AzLvTSz61mUT0TZgkmsMJbYuhHWk06V~bIh3SWNJI7c4wOlzw3dZYQXRp2xEN-wfCnwud5kJJHz0CRIlwmMu4hyvFEZdlUnJQL66-Wvu1PV18mzWSp2tpDABJnpD7dKvZ82utucPl~A9LjLqmTHwEe-Wj3QE0UGwdpqyuhyzwuzGqYIgqYOml8Vhdq2H4eS1K0BMsY9HqJlP45Gh2KrYf7DP32L1zi36K6r1gcRdmlldxaJEL5Zg6B8zZG12j9c~S-cA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. B cell–mediated Th-cell suppression is critically dependent on IL-2. (A-C) Th cells were incubated alone (■), with lgB25+ (▨) or smB25− (▤) cells in presence of cCD3 and different concentrations of IL-2 or without IL-2 (med). (A) [3H]TdR incorporation of T cells. Data are expressed as mean plus or minus SEM of triplicates from 1 representative experiment. (B) PKH-26 assay at day 6 (mean ± SEM of 3 independent experiments). (C) Blockade of IL-2Rαβ by addition of Abs directly to culture or by preincubation of lgB25+ cells (B*) alone; cultures provided with cCD3 and IL-2 (100 U/mL). Mean plus or minus SEM and P value of 5 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-02-140087/7/m_zh80240827520004.jpeg?Expires=1767723250&Signature=4Iq8mSeFW6aE28nTL5zS7F8erzK0tjcmjNPlUjEoXrGdIqB~UC5~jT-HIb-mGHu9BkbleISYrlkfI5vTdVc5mQdSC8zN5P-0LoczgXAw2fkoM1ImPTN1P4heUQUuhHVHKT~XojQKanE69wny~bpcYa46ThoI2gtGwKGGK3HOus9yKfjE-4nVbq1G2uAbV4qN~JVQT2EmktNSgwhX3VCJ4jgfvje7F5uSaP9zBzGsS2v1s9P9rZ1dhJ0t75sRamJ~IUJq~9MkEcPuT1ZYaKng89f7IHSYmYsZGt-4RsUT1MO-7BXUoD1Vm47ZTdehs3zJjZABO~p5KgKJUy3EYBDQBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal