Abstract
Stress or aging of tissue-specific stem cells is considered central to the decline of tissue homeostasis in the elderly, although little is known of molecular mechanisms underlying hematopoietic stem cell (HSC) aging and stress resistance. Here, we report that mice lacking the transcription factor forkhead box O3a (FoxO3a) develop neutrophilia associated with inhibition of the up-regulation of negative regulator of cell proliferation, Sprouty-related Ena/VASP homology 1 domain-containing proteins 2 (Spred2) and AKT and ERK activation, in HSCs during hematopoietic recovery following myelosuppressive stress conditions. Compared with aged wild-type mice, more severe neutrophilia was also observed in aged Foxo3a-deficient mice. AKT and ERK activation and inhibition of Spred2 were detected in HSCs from aged FoxO3a-deficient mice. Spred2-deficient mice also developed neutrophilia during hematopoietic recovery following myelosuppressive stress, indicating that FoxO3a plays a pivotal role in maintenance, integrity, and stress resistance of HSCs through negative feedback pathways for proliferation. This will provide new insight into the hematopoietic homeostasis in conditions of aging and stress.
Introduction
To protect stem cells from various pathophysiological stresses such as oxidative stress, cell cycling, and aging is central to ensure that tissues are functionally sustained throughout an organism's lifetime. Age-dependent stem cell dysfunction may play a pivotal role in the aging process.1,2 Here, we address molecular mechanisms controlling stem cell stress resistance and aging.
Blood cells are continuously consumed and produced from stem cells through progenitor cells. Interestingly, hematopoietic stem cell (HSC) numbers increase substantially with aging,3-5 however, their proliferative and regenerative capacity diminishes with age, an activity largely thought to be cell intrinsic.6 HSCs from aging mice show a skewing of lineage potential from lymphopoiesis toward myelopoiesis correlated with age,3-5,7 and this has been considered due to myeloid lineage bias of aged HSCs,6 though mechanisms underlying this dysfunction and myeloid bias are largely unknown. Hematopoietic homeostasis is sustained by positive and negative regulation. Cytokine signals stimulate events such as differentiation and proliferation and are regulated cell autonomously by several negative feedback loops, such as suppressor of cytokine signaling (SOCS), Sprouty/Spred (for Sprouty-related Ena/VASP homology 1 domain-containing proteins),8-10 and cyclin-dependent kinase (CDK) inhibitors.11-13
Forkhead box (Fox) transcription factors are downstream of the evolutionarily conserved PI3K-AKT pathway, which is activated by growth factors or insulin. FoxO3a is a member of FoxO family, which includes FoxO1, 3, 4, and 6, all of which are PTEN/PI3K/AKT effectors functioning in diverse cellular activities such as induction of cell-cycle arrest, stress resistance, apoptosis, differentiation, and metabolism.14-18 FoxOs also regulate intracellular reactive oxygen species (ROS) levels: loss of FoxOs results in elevated ROS levels, which in turn negatively regulates cellular responses such as erythroid differentiation.19 Recently, it was reported that FoxOs including FoxO3a regulate hematopoietic stem cell function by controlling oxidative stress and cell cycling.20,21 Although FoxO3a is considered downstream of growth factors and AKT signaling,14 a regulatory role of FoxO3a on AKT and ERK has not been demonstrated.
In this study, we show that FoxO3a-deficient mice develop marked neutrophilia with age or during hematopoietic recovery after myelosuppressive stress induced by 5-fluorouracil (5-FU), an antitumor chemotherapeutic agent, in a cell-autonomous manner. AKT and ERK activation were evident in HSCs of 5-FU–treated or aged FoxO3a-deficient mice. FoxO3a-deficient cells were hyperresponsive to cytokine stimulation, a phenotype effectively restored by treatment with AKT-mTOR or a MEK-ERK inhibitor. We found that expression of Spred2 and p27Kip1, both of which inhibit proliferation, was significantly down-regulated in HSCs from 5-FU–treated or aged FoxO3a-deficient mice compared with wild-type mice. Spred2-deficient mice also developed neutrophilia during hematopoietic recovery following myelosuppressive stress, indicating that FoxO3a plays a pivotal role in maintenance, integrity, and stress resistance of HSCs through negative feedback pathways for proliferation.
Methods
Animal procedures
Animal care was in accordance with guidelines for animal and recombinant DNA experiments of the National Institute for Longevity Sciences and Keio University. Foxo3a−/− and Spred2−/− mice were generated as described.20,22 Littermates were used as controls in all experiments. C57BL/6 CD45.1 congenic mice (B6-CD45.1) were purchased from Sankyo-Lab Service (Tsukuba, Japan). NAC was administrated orally (40 mM in drinking water) for 4 weeks and injected subcutaneously (1 g/kg, every 2 days). To analyze blood counts, peripheral blood from the postorbital vein was collected in heparinized microtubes (Drummond Scientific, Broomall, PA) and analyzed using CellTac (Nihon Kohden, Tokyo, Japan). For analysis of blood smears, peripheral blood, or bone marrow, mononuclear cells were stained with May-Gruenwald Giemsa, and the frequency of neutrophils, eosinophils, and basophils was scored. Bone marrow transplantation was performed as described.20 5-FU (Kyowa Hakko, Tokyo, Japan) was administered intravenously at 250 mg/kg to FoxO3a and Spred2 wild-type and null mice and recipient mice received a transplant of FoxO3a+/+ or FoxO3a−/− bone marrow mononuclear cells.
Flow cytometry
Monoclonal antibodies (mAbs) recognizing the following markers were used for flow cytometric analyses and cell sorting: c-Kit (2B8), Sca-1 (E13-161.7), CD4 (L3T4), CD8 (53-6.72), B220 (RA3-6B2), TER-119 (Ly-76), Gr-1 (RB6-8C5), CD34 (RAM34), CD71 (R17217), Ly5.1 (A20), Ly5.2 (104), Mac-1 (M1/70), Ly6G (1A8), and CD3 (500A2). All mAbs were purchased from BD Biosciences (Basel, Switzerland). A mixture of mAbs recognizing CD4, CD8, B220, TER-119, Mac-1, or Gr-1 was used to identify Lineage+ cells.
Long-term cultures and colony-forming assays
Lineage−Sca1+c-Kit+ (LSK) cells were sorted from wild-type or FoxO3a-deficient bone marrow mononuclear cells. Two thousand LSK cells were cocultured with OP9 stromal cells as previously described.23 For some experiments, the NAC (Sigma-Aldrich, St Louis, MO) was added at 100 mM to long-term cultures. After 6-week culture, cells were harvested and used for hematopoietic colony-forming assays. Colony-forming assays were performed with 10 ng/mL G-CSF or 10 ng/mL IL-3 in the methylcellulose medium. After 7 days of culture, the number of colonies was counted. For some experiments, 1 μM rapamycin (Calbiochem, Darmstadt, Germany), 5 μM MEK inhibitor U0126 (Sigma-Aldrich), or 100 μM NAC (Sigma-Aldrich) was added to the culture.
Cytokine enzyme-linked immunosorbent assay
Serum concentrations of mouse G-CSF were determined using highly sensitive enzyme-linked immunosorbent assay (ELISA) kits (Genzyme Techne Analyza Immunoassay System, Cambridge, MA), according to the manufacturer's specification.
Intracellular ROS assay
Real-time PCR analysis
cDNAs were reverse transcribed from total RNA prepared from hematopoietic cells. For quantification, cDNA was subjected to real-time polymerase chain reaction (PCR) using A7500 Fastin Real-Time PCR system (Applied Biosystems, Foster City, CA). GAPDH amplification was used for normalization. Probes and primers used were predesigned transcripts (so-called inventoried assays) validated by Applied Biosystems bioinformatics design pipelines. Applied Biosystems assay IDs were Mm00545913 s1 (SOCS3), Mm00835803 g1 (Spred2), Mm00432448 m1 (p21Cip1), Mm00438167 g1 (p27Kip1), Mm01272133 g1 (p57Kip2), and 4352932E (GAPDH). Each sample was analyzed in triplicate.
Retrovirus preparation
FoxO3a cDNA was PCR amplified and inserted into the pMX-IP vector encoding EGFP to yield pMX-IP-FoxO3a-EGFP. Retrovirus was prepared as described.25 BM mononuclear cells were isolated from wild-type mice and infected by retrovirus in the presence of 10 ng/mL G-CSF or 10 ng/mL GM-CSF. After 7 days, the expression of Gr1 in the PI-negative and EGFP-positive infected cells was analyzed. pMX-IP-EGFP was used as a control.
Immunohistochemical analysis
Mutant and wild-type mice were injected with 5-FU (250 mg/kg) intravenously, and bone marrow mononuclear cells were isolated 2 days later. Cells were stained with FITC-conjugated Lineage markers, PE-conjugated Sca-1, and APC-conjugated c-Kit, and Lineage−Sca-1+ cells were sorted as HSCs by FACS Vantage or Calibur (Becton Dickinson, Franklin Lakes, NJ). Unfractionated bone marrow mononuclear cells or HSCs were then stained with mouse anti–phospho-Akt antibody (Cell Signaling, Danvers, MA) or mouse anti–phospho-ERK antibody (Cell Signaling) followed by Alexa488-conjugated goat anti–mouse Ig antibody, and observed under a confocal microscope (Fluoview FV1000; Olympus, Tokyo, Japan). TOTO3 (Invitrogen, Carlsbad, CA) was used for nuclear staining.
Statistical analysis
P values were calculated using the unpaired Student t test.
Results
Aged FoxO3a-deficient mice develop marked neutrophilia
Initially we examined aged (> 12 months old) wild-type and FoxO3a-deficient mice. Although spontaneous neutrophilia was observed in some wild-type aging mice (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article; and Rossi et al Sudo et al),4,5 neutrophil number was significantly larger in aging FoxO3a-deficient mice compared with age-matched controls (Figure S1), suggesting that FoxO3a negatively regulates age-dependent myeloid expansion in vivo.
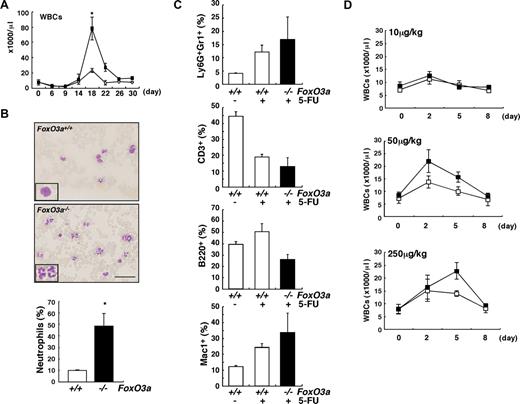
To analyze the role of FoxO3a in hematopoietic homeostasis under in vivo myelosuppressive stress conditions, young (8 to 12 weeks old) FoxO3a-deficient and wild-type mice were treated with 5-FU, which induces apoptosis of actively cycling HSCs and progenitor cells, and recovery of peripheral blood was analyzed (Figure 1). Although levels of hemoglobin and numbers of platelets recovered normally in mutant mice (Figure S2), the number of white blood cells (WBCs), especially neutrophils, increased markedly in FoxO3a-deficient mice during hematopoietic recovery after 5-FU–induced myelosuppression (Figure 1A,B). There was no increase in eosinophils and basophils (data not shown). The frequency of Ly6G, a marker of neutrophils, positive cells in Gr1+ cells, or Mac1+ cells, increased in peripheral blood of FoxO3a-deficient mice during hematopoietic recovery after 5-FU treatment (Figure 1C). FoxO3a-deficient mice developed neutrophilia during recovery from 5-FU treatment, suggesting that FoxO3a might negatively regulate granulopoiesis. Consistent with the neutrophilia observed in FoxO3a-deficient mice, an increased number and proportion of granulocytes accumulated in bone marrow after 5-FU treatment (data not shown). In contrast, the frequency of CD3+ T cells or B220+ B cells decreased in peripheral blood of FoxO3a-deficient mice during hematopoietic recovery after 5-FU treatment (Figure 1C). Administration of granulocyte colony-stimulating factor (G-CSF) in Foxo3a-deficient mice significantly increased the peripheral WBC count, even at a low G-CSF concentration (Figure 1D). These results suggest that FoxO3a-deficient mice are hyperresponsive to G-CSF during mobilization of neutrophils.
FoxO3a-deficient mice develop neutrophilia following 5-FU injection. (A) A drastic increase in WBC count was detected FoxO3a-deficient mice 18 days after 5-FU treatment. ■ indicates WBC counts in FoxO3a-deficient mice (n = 10); and ○, WBC counts in wild-type mice (n = 10). Data are mean counts (×1000/μL) plus or minus SD of WBCs in FoxO3a-deficient or wild-type mice. *P < .01. (B) (Top panels) Peripheral blood images stained with May-Gruenwald Giemsa obtained from 8- to 12-week-old FoxO3a-deficient or wild-type mice 18 days after 5-FU treatment (inset, high magnification). (Bottom panel) Data are mean frequency (%) plus or minus SD of neutrophils determined by May-Gruenwald Giemsa staining in peripheral WBCs from FoxO3a-deficient (■; n = 5) or wild-type (□; n = 5) mice 18 days after 5-FU (250 mg/kg) treatment. *P < .01. Bar represents 100 μm. (C) Peripheral blood mononuclear cells were isolated from 8- to 12-week-old FoxO3a-deficient (■; n = 4) or wild-type (□; n = 3) mice 17 days after 5-FU treatment, and the expression of Ly6G, Gr1, CD3, B220, and Mac1 was analyzed by FACS. Data are mean frequency (%) plus or minus SD of indicated population in peripheral blood mononuclear cells. (D) Peripheral WBC counts in 8- to 12-week-old FoxO3a-deficient or wild-type mice administered subcutaneously with indicated G-CSF concentrations. ■ indicates WBC counts in FoxO3a-deficient mice (n = 3); and □, WBC counts in wild-type mice (n = 3). Data are mean counts (×1000/μL) plus or minus SD of WBCs.
FoxO3a-deficient mice develop neutrophilia following 5-FU injection. (A) A drastic increase in WBC count was detected FoxO3a-deficient mice 18 days after 5-FU treatment. ■ indicates WBC counts in FoxO3a-deficient mice (n = 10); and ○, WBC counts in wild-type mice (n = 10). Data are mean counts (×1000/μL) plus or minus SD of WBCs in FoxO3a-deficient or wild-type mice. *P < .01. (B) (Top panels) Peripheral blood images stained with May-Gruenwald Giemsa obtained from 8- to 12-week-old FoxO3a-deficient or wild-type mice 18 days after 5-FU treatment (inset, high magnification). (Bottom panel) Data are mean frequency (%) plus or minus SD of neutrophils determined by May-Gruenwald Giemsa staining in peripheral WBCs from FoxO3a-deficient (■; n = 5) or wild-type (□; n = 5) mice 18 days after 5-FU (250 mg/kg) treatment. *P < .01. Bar represents 100 μm. (C) Peripheral blood mononuclear cells were isolated from 8- to 12-week-old FoxO3a-deficient (■; n = 4) or wild-type (□; n = 3) mice 17 days after 5-FU treatment, and the expression of Ly6G, Gr1, CD3, B220, and Mac1 was analyzed by FACS. Data are mean frequency (%) plus or minus SD of indicated population in peripheral blood mononuclear cells. (D) Peripheral WBC counts in 8- to 12-week-old FoxO3a-deficient or wild-type mice administered subcutaneously with indicated G-CSF concentrations. ■ indicates WBC counts in FoxO3a-deficient mice (n = 3); and □, WBC counts in wild-type mice (n = 3). Data are mean counts (×1000/μL) plus or minus SD of WBCs.
Cell-autonomous regulation of granulopoiesis by FoxO3a
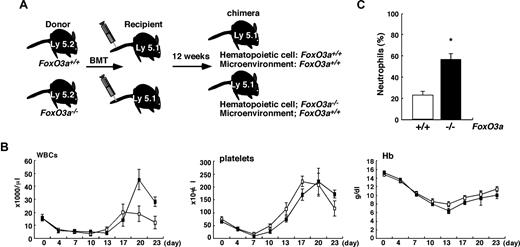
To exclude the possibility a stem cell niche such as sinusoidal endothelial cells might function in promoting HSC recruitment and hematopoietic recovery after BM suppression, we analyzed mice that received a transplant of BM cells isolated from FoxO3a-deficient or wild-type mice (Figure 2). The rate of chimerism seen in transplanted cells in peripheral blood was more than 90% in each type of recipient mouse 12 weeks after transplantation (data not shown). Recipients were treated with 5-FU, and peripheral blood counts were scored. Although levels of hemoglobin and numbers of platelets recovered normally, dramatic increases in the number of WBCs, especially neutrophils, were observed in transplant recipients of FoxO3a-deficient cells compared with mice that received a transplant of wild-type cells, indicating that transplant recipients of FoxO3a-deficient cells developed neutrophilia during hematopoietic recovery after BM suppression (Figure 2B,C).
Cell-autonomous regulation of granulopoiesis by FoxO3a. (A) A schematic experimental procedure is shown. BMT indicates bone marrow transplantation. (B) Peripheral blood counts in mice that received a transplant of wild-type (□; n = 5) or FoxO3a-deficient (■; n = 5) cells following 5-FU treatment. Data are means (×1000/μL) plus or minus SD of WBCs (×1000/μL), plus or minus SD of platelets, and (grams per deciliter) plus or minus SD of Hb. (C) Mice that received a transplant of FoxO3a-deficient cells develop neutrophilia following 5-FU injection. The peripheral blood smear was stained with May-Gruenwald Giemsa, and the frequency of neutrophils in peripheral blood was determined. Data are mean frequency (%) plus or minus SD of neutrophils in peripheral WBCs of mice that received a transplant of FoxO3a-deficient (■; n = 5) or wild-type (□; n = 5) cells 18 days after 5-FU injection. *P < .01.
Cell-autonomous regulation of granulopoiesis by FoxO3a. (A) A schematic experimental procedure is shown. BMT indicates bone marrow transplantation. (B) Peripheral blood counts in mice that received a transplant of wild-type (□; n = 5) or FoxO3a-deficient (■; n = 5) cells following 5-FU treatment. Data are means (×1000/μL) plus or minus SD of WBCs (×1000/μL), plus or minus SD of platelets, and (grams per deciliter) plus or minus SD of Hb. (C) Mice that received a transplant of FoxO3a-deficient cells develop neutrophilia following 5-FU injection. The peripheral blood smear was stained with May-Gruenwald Giemsa, and the frequency of neutrophils in peripheral blood was determined. Data are mean frequency (%) plus or minus SD of neutrophils in peripheral WBCs of mice that received a transplant of FoxO3a-deficient (■; n = 5) or wild-type (□; n = 5) cells 18 days after 5-FU injection. *P < .01.
The serum G-CSF concentration of FoxO3a-deficient mice before and after 5-FU treatment was equivalent to that of 5-FU–treated wild-type mice (Figure S3). Although expression levels of the G-CSF receptor were comparable in mutant or wild-type cells (data not shown), the number of colonies formed by BM mononuclear cells in the presence of G-CSF was significantly greater in FoxO3a-deficient cells than wild-type cells at any G-CSF concentration (Figure S4). Furthermore, overexpression of FoxO3a inhibited granulopoiesis induced by G-CSF or GM-CSF (Figure S5). These results indicate that FoxO3a negatively regulates myeloid, especially neutrophil, expansion cell autonomously.
Several studies including ours have shown that FoxOs regulate intracellular ROS levels, and that FoxO-deficient cells are defective in various cellular responses to oxidative stress.19-21 Defective HSC function and erythropoiesis were reportedly rescued by treatment with the antioxidant N-acetylcysteine (NAC) in FoxO-null and FoxO3a-deficient mice, respectively.19,21 However, NAC treatment of 5-FU–treated FoxO3a-deficient mice did not inhibit development of neutrophilia (Figure S6). Indeed, intracellular ROS levels did not differ between FoxO3a-deficient and wild-type HSCs following 5-FU treatment (Figure S6), suggesting that FoxO3a regulates granulopoiesis via a mechanism different from controlling intracellular ROS levels. Defective HSC function was seen in FoxO3a-deficient HSCs by long-term culture assays (LTC-IC), however, it was partially but significantly restored by a NAC treatment (Figure S6), suggesting that the defect of HSC function in FoxO3a-deficient mice was due, at least in part, to the defect of controlling intracellular oxidative stress.
AKT is hyperphosphorylated in FoxO3a-deficient HSCs following 5-FU treatment
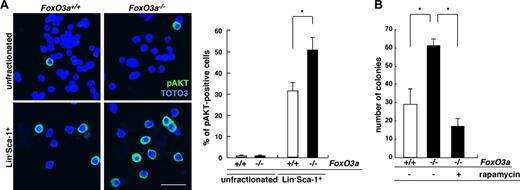
FoxO3a protein is nuclear in quiescent HSCs and confined to the cytoplasm following stimuli with cytokines.20,26 Since FoxO3a is a transcription factor, we hypothesized that it may regulate granulopoiesis in HSCs by remaining nuclear. c-Kit–positive compartment was obliterated in Lineage− fraction of 5-FU–treated mice and HSCs were preserved in the fraction of Lineage−Sca-1+ (Lin−Sca-1+) cells following 5-FU treatment. Lin−Sca-1+ cells or unfractionated bone marrow (BM) mononuclear cells were isolated from 5-FU–treated FoxO3a-deficient or wild-type mice and analyzed for AKT activation, which occurs following cytokine stimulation (Figure 3). The percentage of cells positive for AKT phosphorylation did not differ between unfractionated BM cells from 5-FU–treated FoxO3a-deficient and wild-type mice (Figure 3A). However, AKT was strongly activated in HSCs, particularly among BM cells that survived 5-FU treatment, and interestingly, the proportion of cells positive for phosphorylated AKT was significantly up-regulated in FoxO3a-deficient HSCs compared with wild-type HSCs (Figure 3A). In addition, hyperresponsivity to G-CSF seen in FoxO3a-deficient cells was significantly down-regulated by treatment with rapamycin, which inhibits the AKT target mTOR (Figure 3B). These observations suggest that AKT hyperactivation seen in FoxO3a-deficient HSCs accounts in part for neutrophilia seen in FoxO3a-deficient mice during hematopoietic recovery following 5-FU treatment, although FoxO3a is apparently downstream of AKT.
AKT is activated in HSCs of FoxO3a-deficient mice during hematopoietic recovery after 5-FU–induced myelosuppressive stress. (A) (Left panel) Phospho-AKT immunostaining in BM mononuclear cells (unfractionated) or Lin−Sca-1+ cells 2 days after 5-FU injection in 8- to 12-week-old FoxO3a-deficient or wild-type mice. (Right panel) Data mean frequency (%) plus or minus SD of phospho-AKT–positive cells in Lin−Sca-1+ cells (n = 5). *P < .01. Bar represents 100 μm. (B) FoxO3a-deficient cells are hyperresponsive to G-CSF, a phenotype rescued by rapamycin treatment. Results are means plus or minus SD of the number of colonies (n = 5). *P < .01.
AKT is activated in HSCs of FoxO3a-deficient mice during hematopoietic recovery after 5-FU–induced myelosuppressive stress. (A) (Left panel) Phospho-AKT immunostaining in BM mononuclear cells (unfractionated) or Lin−Sca-1+ cells 2 days after 5-FU injection in 8- to 12-week-old FoxO3a-deficient or wild-type mice. (Right panel) Data mean frequency (%) plus or minus SD of phospho-AKT–positive cells in Lin−Sca-1+ cells (n = 5). *P < .01. Bar represents 100 μm. (B) FoxO3a-deficient cells are hyperresponsive to G-CSF, a phenotype rescued by rapamycin treatment. Results are means plus or minus SD of the number of colonies (n = 5). *P < .01.
ERK is hyperphosphorylated in FoxO3a-deficient HSCs following 5-FU treatment
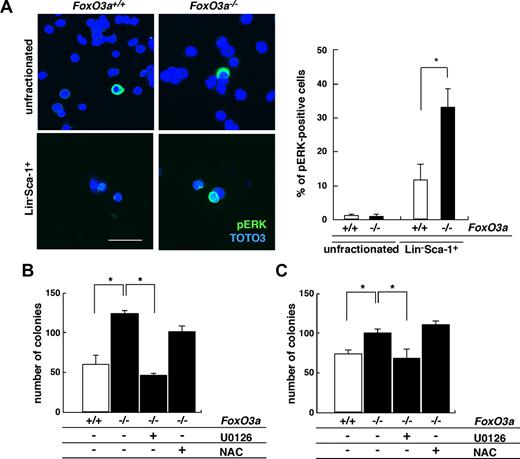
Cytokine/growth factor stimulation can mediate cell proliferation via MEK-ERK activation. We analyzed ERK activation in HSCs from 5-FU–treated, FoxO3a-deficient mice (Figure 4). Similar to AKT, ERK phosphorylation was significantly up-regulated in HSCs among surviving BM cells from 5-FU–treated mice, and interestingly, ERK activation was also significantly up-regulated in FoxO3a-deficient HSCs compared with wild-type HSCs (Figure 4A). Moreover, the hyperresponsive to G-CSF treatment seen in FoxO3a-deficient cells was suppressed to basal levels by treatment with U0126, a specific MEK inhibitor, thereby inhibiting ERK activation (Figure 4B). FoxO3a-deficient cells were also hyperresponsive to IL-3 compared with wild-type cells, and again, this response was restored by U0126 treatment (Figure 4C). However, excess proliferation of FoxO3a-deficient cells in the presence of G-CSF or IL-3 was not rescued by antioxidant treatment (Figure 4B,C). These results indicate that FoxO3a negatively regulates granulopoiesis by inhibiting AKT and ERK pathways in HSCs.
ERK is phosphorylated in HSCs of FoxO3a-deficient mice during hematopoietic recovery after 5-FU–induced myelosuppressive stress conditions. (A) (Left panel) Phospho-ERK immunostaining in BM mononuclear cells (unfractionated) or Lin−Sca-1+ cells 2 days after 5-FU injection in 8- to 12-week-old FoxO3a-deficient or wild-type mice. (Right panel) Data are mean frequency (%) plus or minus SD of phospho-ERK–positive cells in Lin−Sca-1+ cells (n = 5). *P < .01. Bar represents 100 μm. (B,C) FoxO3a-deficient cells are hyperresponsive to G-CSF (B) and IL-3 (C) and their phenotypes are restored by U0126 but not NAC treatment. Bone marrow mononuclear cells were isolated from wild-type and FoxO3a-deficient mice and cultured with 10 ng/mL G-CSF or 10 ng/mL IL-3 in methylcellulose medium for 7 days; then the number of colonies was scored. □ indicates wild-type cells (n = 5); and ■, FoxO3a-deficient cells (n = 5). Results are mean plus or minus SD of the number of colonies. *P < .01.
ERK is phosphorylated in HSCs of FoxO3a-deficient mice during hematopoietic recovery after 5-FU–induced myelosuppressive stress conditions. (A) (Left panel) Phospho-ERK immunostaining in BM mononuclear cells (unfractionated) or Lin−Sca-1+ cells 2 days after 5-FU injection in 8- to 12-week-old FoxO3a-deficient or wild-type mice. (Right panel) Data are mean frequency (%) plus or minus SD of phospho-ERK–positive cells in Lin−Sca-1+ cells (n = 5). *P < .01. Bar represents 100 μm. (B,C) FoxO3a-deficient cells are hyperresponsive to G-CSF (B) and IL-3 (C) and their phenotypes are restored by U0126 but not NAC treatment. Bone marrow mononuclear cells were isolated from wild-type and FoxO3a-deficient mice and cultured with 10 ng/mL G-CSF or 10 ng/mL IL-3 in methylcellulose medium for 7 days; then the number of colonies was scored. □ indicates wild-type cells (n = 5); and ■, FoxO3a-deficient cells (n = 5). Results are mean plus or minus SD of the number of colonies. *P < .01.
Spred2 regulates granulopoiesis downstream of FoxO3a
To clarify potential mechanisms of ERK hyperactivation in HSCs of FoxO3a-deficient mice, we analyzed expression of Spred2, a negative regulator of growth factor– and cytokine-induced MEK-ERK signals.8 Spred2 expression was significantly lower in FoxO3a-deficient HSCs than in wild-type HSCs (Figure 5A); however, expression of SOCS3, another negative regulator of cytokine signals and granulopoiesis,9,27,28 did not differ between FoxO3a-deficient and wild-type HSCs (Figure 5A). Similar expression patterns were seen in FoxO3a-deficient cells transplanted into recipient mice (Figure S7), whereas Spred2 up-regulation was induced in FoxO3a-overexpressing cells (data not shown).
Spred2 is downstream of FoxO3a and mediates granulopoiesis. (A) Spred2 but not SOCS3 expression in Lin−Sca1+c-Kit+ (LSK) cells was down-regulated in FoxO3a-deficient mice. Data are means plus or minus SD of Spred2 or SOCS3 expression relative to GAPDH in FoxO3a-deficient LSK cells (■; n = 5) relative to wild-type LSK (□; n = 5) cells. *P < .01. (B) Spred2-deficient mice develop granulopoiesis during hematopoietic recovery following 5-FU injection. Data shown are means plus or minus SD of WBCs (×1000/μL), platelets (×104/μL), or Hb (g/dL) of Spred2-deficient (■; n = 5) or wild-type (□; n = 5) mice. (C,D) AKT and ERK are activated in HSCs of FoxO3a-deficient mice during hematopoietic recovery after 5-FU–induced myelosuppressive stress. BM mononuclear cells (unfractionated) or Lin−Sca-1+ cells were isolated from 8- to 12-week-old Spred2-deficient or wild-type mice 2 days after 5-FU injection and stained with phospho-AKT (C) or phospho-ERK (D), and the frequency of phospho-AKT (C)– or phospho-ERK (D)–positive cells was determined. Data are mean frequency (%) plus or minus SD of phospho-AKT or ERK-positive cells. *P < .05.
Spred2 is downstream of FoxO3a and mediates granulopoiesis. (A) Spred2 but not SOCS3 expression in Lin−Sca1+c-Kit+ (LSK) cells was down-regulated in FoxO3a-deficient mice. Data are means plus or minus SD of Spred2 or SOCS3 expression relative to GAPDH in FoxO3a-deficient LSK cells (■; n = 5) relative to wild-type LSK (□; n = 5) cells. *P < .01. (B) Spred2-deficient mice develop granulopoiesis during hematopoietic recovery following 5-FU injection. Data shown are means plus or minus SD of WBCs (×1000/μL), platelets (×104/μL), or Hb (g/dL) of Spred2-deficient (■; n = 5) or wild-type (□; n = 5) mice. (C,D) AKT and ERK are activated in HSCs of FoxO3a-deficient mice during hematopoietic recovery after 5-FU–induced myelosuppressive stress. BM mononuclear cells (unfractionated) or Lin−Sca-1+ cells were isolated from 8- to 12-week-old Spred2-deficient or wild-type mice 2 days after 5-FU injection and stained with phospho-AKT (C) or phospho-ERK (D), and the frequency of phospho-AKT (C)– or phospho-ERK (D)–positive cells was determined. Data are mean frequency (%) plus or minus SD of phospho-AKT or ERK-positive cells. *P < .05.
These observations led us to analyze the role of Spred2 in granulopoiesis after myelosuppression by 5-FU treatment. Adult Spred2-deficient mice are apparently healthy and show no overt abnormalities.22 We treated Spred2-deficient mice and control littermates with 5-FU and analyzed levels of hemoglobin and numbers of WBCs and platelets (Figure 5B). Spred2-deficient mice also showed WBC-specific overproliferation during hematopoietic recovery following myelosuppression by 5-FU treatment (Figure 5B). The hyperphosphorylation of AKT and ERK was also detected in Spred2-deficient HSCs compared with wild-type HSCs during hematopoietic recovery after myelosuppression (Figure 5C.D). These results suggest that Spred2 down-regulation in FoxO3a-deficient HSCs hyperactivates ERK and results in hyperproliferation of neutrophils.
The CDK inhibitor p27Kip1 is down-regulated in FoxO3a-deficient HSCs following 5-FU treatment
To further clarify potential mechanisms underlying hyperproliferation of granulocytes in FoxO3a-deficient mice following 5-FU treatment, we analyzed expression of CDK inhibitors, which negatively regulate cell proliferation. The CDK inhibitor p27Kip1 was dramatically activated in HSCs, specifically among BM cell survivors of 5-FU treatment in wild-type mice, suggesting activation of a negative feedback loop (Figure S8A). Interestingly, up-regulation of p27Kip1 mRNA in FoxO3a-deficient HSCs was significantly inhibited compared with wild-type HSCs following 5-FU–induced stress, suggesting that FoxO3a-deficient HSCs lack a negative feedback pathway that antagonizes overproliferation (Figure S8A). In contrast, p21Cip1 expression was highly activated in FoxO3a-deficient HSCs in both mRNA and protein levels (Figure S8B). Interestingly, protein p57Kip2 expression was up-regulated in both wild-type and FoxO3a-deficient HSCs compared with unfractionated BM cells, however, the mRNA levels for p57Kip2 were not different between HSCs and unfractionated BM cells (Figure S8C), suggesting that proteolytic activity to p57Kip2 might be down-regulated in HSCs under stress condition following myelosuppression.
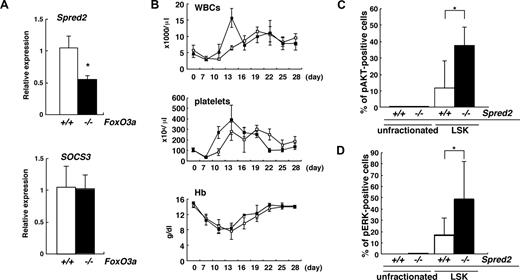
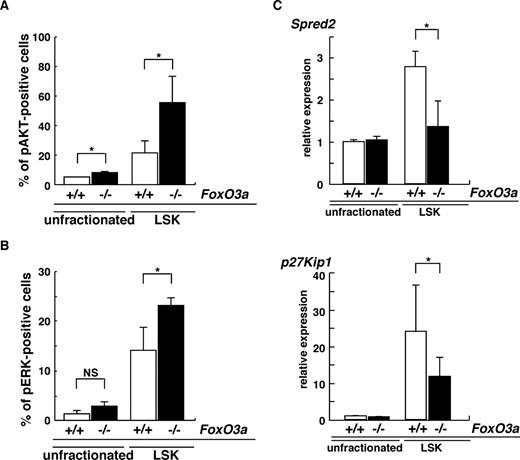
Spontaneous neutrophilia was observed in aging FoxO3a-deficient and wild-type mice (Figure S1). However, since aging FoxO3a-deficient mice develop more severe neutrophilia than aging wild-type mice (Figure S1), we hypothesized that HSC phenotypes seen in aging FoxO3a-deficient mice may resemble those of HSCs from 5-FU–treated FoxO3a-deficient mice. Indeed, hyperactivation of both AKT and ERK was detected in immature hematopoietic cell populations: Lineage−Sca-1+c-Kit+ (LSK) cells derived from aged FoxO3a-deficient mice compared with wild-type mice (Figure 6A,B). Spred2 expression was significantly down-regulated in LSK cells from aged FoxO3a-deficient mice compared with wild-type mice (Figure 6C). p27Kip1 mRNA expression was also dramatically up-regulated in LSK cells compared with unfractionated BM cells from aged wild-type mice; however, p27Kip1 up-regulation in LSK cells from aged FoxO3a-deficient mice was significantly inhibited compared with that seen in wild-type mice (Figure 6C). These results were similar to those seen in HSCs from 5-FU–treated FoxO3a-deficient mice. Interestingly, a marked increase in WBCs was observed in old mice (12 to 18 months old) compared with younger mice (8 to 12 weeks old) during hematopoietic recovery after 5-FU–induced myelosuppression (Figure S9). Taken together, our results indicate that FoxO3a plays a critical role in regulating HSC function and integrity under stressed and aging conditions by inhibiting hyperproliferation via negative feedback pathways.
HSCs in aged FoxO3a-deficient mice are phenotypically similar to HSCs in FoxO3a-deficient mice injected with 5-FU. (A,B) Data are mean frequencies (%) plus or minus SD of phospho-AKT (A)– or ERK (B)–positive cells in LSK cells from aged FoxO3a+/+ (□; n = 3) or FoxO3a−/− (■; n = 3) cells. *P < .05. (C) Spred2 and p27Kip1 are down-regulated in LSK cells from aged FoxO3a-deficient mice. Data are means plus or minus SD of Spred2 and p27Kip1 expression relative to GAPDH in LSK or unfractionated cells from aged FoxO3a-deficient mice (■; n = 3) relative to unfractionated cells from aged wild-type mice (□; n = 3). *P < .01.
HSCs in aged FoxO3a-deficient mice are phenotypically similar to HSCs in FoxO3a-deficient mice injected with 5-FU. (A,B) Data are mean frequencies (%) plus or minus SD of phospho-AKT (A)– or ERK (B)–positive cells in LSK cells from aged FoxO3a+/+ (□; n = 3) or FoxO3a−/− (■; n = 3) cells. *P < .05. (C) Spred2 and p27Kip1 are down-regulated in LSK cells from aged FoxO3a-deficient mice. Data are means plus or minus SD of Spred2 and p27Kip1 expression relative to GAPDH in LSK or unfractionated cells from aged FoxO3a-deficient mice (■; n = 3) relative to unfractionated cells from aged wild-type mice (□; n = 3). *P < .01.
Discussion
Homeostasis of several adult tissues is determined, at least in part, by the integrity of tissue-specific stem cells.6 Stem cells provide both daughter self-renewing cells and progenitors that differentiate into mature cells through an organism's lifespan. Stem cells must be protected from pathophysiological stresses for a lifetime. Here, we show that aging and myelosuppressive/growth-stimulating stress induce similar changes in FoxO3a-deficient HSCs, and that FoxO3a plays a central role in regulating maintenance of HSC function under conditions of stress and aging.
FoxO factors regulate HSC oxidative stress and cell cycling, thereby regulating the HSC pool and its function in mammals.19-21 Here, we demonstrate a novel role of FoxO3a in regulating HSC function by negatively regulating AKT and ERK activation pathways. Loss of FoxO3a results in development of neutrophilia during hematopoietic recovery after myelosuppression, or in aging, suggesting that FoxO3a regulates the number of neutrophils and limits overproliferation. Expansion of myeloid lineage-specific cells has been observed as a normal consequence of aging.5 Such expansion is more drastic in aged FoxO3a-deficient than wild-type mice, indicating that FoxO3a regulates an age-dependent myeloid bias. Since neutrophilia was also induced in wild-type mice that received a transplant of FoxO3a-deficient cells, FoxO3a regulates granulopoiesis cell autonomously rather than by modulating microenvironments. Indeed, hyperresponsivity to cytokine stimulation was observed in FoxO3a-deficient cells, even in vitro. These lines of evidence indicate that FoxO3a negatively regulates the cell cycle and that loss of this activity results in hyperoscillation of blood cells, which may exhaust the HSC pool. Indeed, we previously reported that FoxO3a-deficient mice are more sensitive to serial 5-FU treatment than are wild-type mice.20
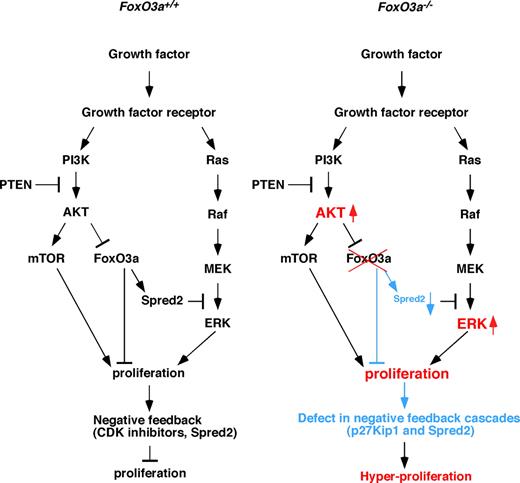
FoxO3a is a target of PI3-kinase and AKT signaling, and growth factor–stimulated activation of the PI3-kinase/AKT pathway blocks FoxO3a-dependent transcription. In the absence of FoxO3a, HSCs cycle, indicating that FoxO3a regulates HSC quiescence.20 Overexpression or conditional activation of FoxO factors antagonizes constitutive PI3K/PKB activation, including effects on cell proliferation, indicating that FoxOs are downstream of AKT signaling.14 We observed that AKT was highly activated in HSCs from 5-FU–treated and aged FoxO3a−/− mice compared with wild type-mice, suggesting that FoxO3a regulates AKT activation via a negative feedback pathway (Figure 7). Similar to AKT, the ERK pathway was also highly activated in HSCs from stressed and aged FoxO3a−/− mice compared with wild-type mice, and treatment with an AKT-mTOR or MEK-ERK inhibitor effectively rescued hyperresponsivity to cytokines in FoxO3a-deficient cells. These results suggest that a negative feedback loop is linked to cell proliferation. In our study, expression of Spred2 and p27Kip1, both of which negatively regulate the cell cycle, was down-regulated in HSCs from stressed and aged FoxO3a-deficient mice, indicating that FoxO3a regulates cellular quiescence by activating these pathways (Figure 7). Thus FoxO3a regulates AKT and ERK activation through Spred2 and p27Kip1 in a negative feedback loop (Figure 7 left panel). Loss of FoxO3a lacks negative feedback loops, which results in a hyperproliferation of HSCs (Figure 7 right panel). Since AKT activation and FoxO inactivation occur in various human cancers, both events may cooperatively regulate cell proliferation.29,30
Regulation of cell proliferation by FoxO3a. (Left) Growth factor signals in HSCs of stressed or aged wild-type mice. Growth factor signals activate both AKT-mTOR and MEK-ERK pathways. These pathways cooperatively stimulate cell proliferation, in turn up-regulating Spred2 and CDK inhibitors to oppose proliferation through negative feedback. (Right) Growth factor signals in HSCs of stressed or aged FoxO3a-deficient mice. In the absence of FoxO3a, negative feedback pathways up-regulating Spred2 and p27Kip1 expression and antagonizing proliferation are strongly inhibited in HSCs, and AKT and ERK are hyperactivated, resulting in myeloproliferation.
Regulation of cell proliferation by FoxO3a. (Left) Growth factor signals in HSCs of stressed or aged wild-type mice. Growth factor signals activate both AKT-mTOR and MEK-ERK pathways. These pathways cooperatively stimulate cell proliferation, in turn up-regulating Spred2 and CDK inhibitors to oppose proliferation through negative feedback. (Right) Growth factor signals in HSCs of stressed or aged FoxO3a-deficient mice. In the absence of FoxO3a, negative feedback pathways up-regulating Spred2 and p27Kip1 expression and antagonizing proliferation are strongly inhibited in HSCs, and AKT and ERK are hyperactivated, resulting in myeloproliferation.
Inhibiting cell-cycle progression in HSCs is essential to maintain HSC quiescence. CDK inhibitors, especially p21Cip1, regulate HSC function by antagonizing proliferation and are therefore considered crucial to control HSC function. We found that in wild-type mice p27Kip1, p21Cip1, and p57Kip2 were all activated in HSCs specifically among BM cell survivors of 5-FU treatment; however, p27Kip1 up-regulation was significantly inhibited in HSCs from stressed FoxO3a−/− mice compared with wild-type mice (Figure S8). These results suggest that reduced p27Kip1 activation plays, at least in part, a role in neutrophilia seen in FoxO3a-deficient mice following stress induced by chemotherapeutic treatment. Taken together, our results demonstrate that FoxO3a plays a critical role in regulating HSC function under stressed and aging conditions by antagonizing AKT and ERK pathways via negative feedback.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Y. Sato for technical support. Retroviral vectors were provided by Dr T. Kitamura (Institute of Medical Science, University of Tokyo, Tokyo, Japan).
This study was supported by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO), Ibaraki, Japan (A.Y.). T.M. was supported by Precursory Research for Embryonic Science and Technology (PREST), Kawaguchi, Japan. T.S. was supported by a grant-in-aid from Specially Promoted Research of the Ministry of Education, Science, Sports and Culture, Tokyo, Japan.
Authorship
Contribution: K.M. and R.K. performed research and analyzed data; T.M. designed and performed research, and wrote the paper; A.Y. and N.M. designed research; T.S. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takeshi Miyamoto or Toshio Suda, Department of Cell Differentiation, Keio University School of Medicine, 35 Shinano-machi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail: miyamoto@sc.itc.keio.ac.jp or sudato@sc.itc.keio.ac.jp.
References
Author notes
*K.M. and T.M. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal