Drawing from initial findings in NOX2/NADPH oxidase-deficient mice, Mantegazza and colleagues show in this issue of Blood that NOX2 plays a key role in antigen crosspresentation by human DCs. This study provides further support for a role of NOX2 in adaptive immunity and possibly in the maintenance of tolerance.
Crosspresentation is the phenomenon by which antigens are processed by antigen-presenting cells and presented to CD8+ T lymphocytes on MHC/HLA class I molecules.1,2 Crosspresentation is primarily performed by dendritic cells (DCs) and is likely to be an essential mechanism for the initiation of cytotoxic T-cell responses as well as for tolerance maintenance.3 Initially reported by Michael J. Bevan back in 1975, crosspresentation has remained a mystery in terms of the mechanisms allowing this form of antigen processing to occur. But in recent years, some insights have shed light on this pathway4
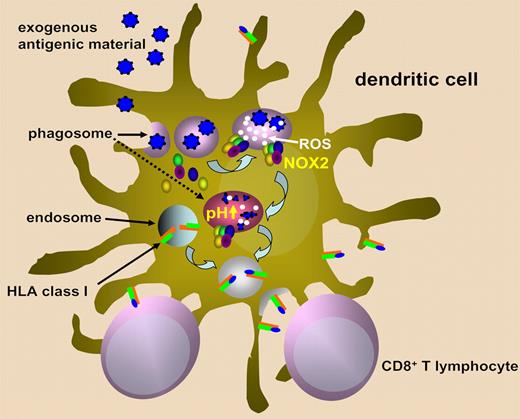
The current model of crosspresentation entails the fusion of early phagosomes with the endoplasmic reticulum, leading to hybrid organelles that contain all the required components for HLA class I molecule assembly including the transporter associated with antigen presentation (TAP), calreticulin, and ERp57. From here, phagocytosed proteins would be transported to the cytosol to undergo proteasome-mediated degradation, and then the generated antigen-derived peptides would be transported back into the phagosome to be loaded onto HLA class I molecules.1,2,5,6
NADPH oxidase/NOX2 is an enzymatic complex that is normally unassembled in resting cells but assembles and becomes activated at the phagosomal membrane following cell stimulation.7 NOX2 subunits include, among others, cytochrome b558, which, in turn, is constituted by gp91phox and p22phox. NOX2 produces superoxide anions, which are converted to hydrogen peroxide and more reactive oxygen species (ROS). ROS generation via NOX2 consumes large amounts of protons and thereby causes a strong alkalinization of the phagosome lumen that may reach pH 8. The effect of NOX2 on phagosomal pH is antagonized by vacuolar ATPase (V-ATPase), which transports protons from the cytosol into the lumen of phagosomes and is responsible for the acidification of this compartment.
Using gp91phox-/- mice, Savina and colleagues had previously shown that NOX2 is recruited to early phagosomes in DCs where it mediates the production of low levels of ROS, causing active and sustained phagosome alkalinization.7 NOX2-deficient mouse DCs showed enhanced phagosomal acidification and increased antigen degradation, which were detrimental for crosspresentation. The authors suggested that pHs above 7 (the natural pHs in DC phagosomes) may be optimal for crosspresentation. On the contrary, pHs lower than 6.5 (as observed in NOX2-deficient DCs) may lead to inefficient crosspresentation, probably by triggering excessive protease activation.
In their report, Mantegazza et al make use of the NOX2 inhibitor DPI and of DCs generated from individuals affected by chronic granulomatous disease (CGD; a disease where one or more NOX2 components are absent or dysfunctional) to show that NOX2 is involved in phagosome alkalinization in human (monocyte-derived) DCs too. As a consequence, in line with previous findings, DCs treated with DPI or DCs from CGD patients show impaired crosspresentation of tumor antigens to CD8+ T cells. Since crosspresentation also has a role in tolerance-maintenance, the authors speculate that the increased propensity to autoimmune manifestations observed in CGD patients could be due to defective crosspresentation of self antigens at the time of negative selection.
Savina et al proposed that phagosome pH has a crucial role in determining “if this compartment is more adapted to kill or to process.”7 In macrophages, phagosome acidification is highly efficient as it aims for a maximal activation of proteases to facilitate microbe killing. This goal is achieved here by expressing high V-ATPase levels. Conversely, antigen crosspresentation in DCs appears to require some protein degradation to generate antigenic peptides, but not so much as to completely destroy all peptides and loss of potential T-cell epitopes. NOX2 may come into play at this point to fine tune phagosomal pH and thereby confer DCs the ability to process proteins instead of killing microbes (see figure).
Schematic representation of the role of NOX2 in ROS generation, phagosome alkalinization, and presentation of exogenous antigens on class I HLA molecules.
Schematic representation of the role of NOX2 in ROS generation, phagosome alkalinization, and presentation of exogenous antigens on class I HLA molecules.
The observations of Mantegazza et al leave several open questions. In the first place, it remains to be explained exactly how NOX2-derived ROS and phagosome alkalinization promote crosspresentation. It would then be interesting to see if NOX2 expression/activity changes between immunogenic versus tolerogenic DCs and whether modulating NOX2 activity may be a viable strategy to promote crosspriming against infectious or cancer antigens, or vice versa, to avoid autoimmunity in predisposed subjects. The appealing speculation that a defect in crosspresentation may contribute to autoimmunity in CGD patients requires more investigation.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal