In this issue of Blood, Cao and colleagues show that HO-1 plays a key role in the maintenance of HSCs by limiting the stress-induced proliferation of progenitors and the exhaustion of HSC populations.
Heme is an essential molecule in a wide variety of biological processes, including the transport and detection of oxygen and other diatomic gases and several forms of catalysis that depend on election transfer. Heme can also be highly toxic due to its redox potential, and therefore, its levels are tightly regulated in all cells. The degradation of heme is an important part of this regulation, and it is carried out by 2 ubiquitously expressed heme-oxygenases that convert the molecule into carbon monoxide (CO), iron, and biliverdin (which is later converted to bilirubin). Heme-oxygenase 1 (HO-1) is the inducible isoform and is increased in response to oxidative stress, hypoxia, heavy metals, and several inflammatory cytokines. HO-1 also helps to maintain the steady-state level of heme through a feedback loop in which heme binds and inhibits a HO-1 transcriptional repressor, Bach1.1 In contrast, heme-oxygenase 2 (HO-2) is a constitutive isoform, which is expressed under homeostatic conditions.
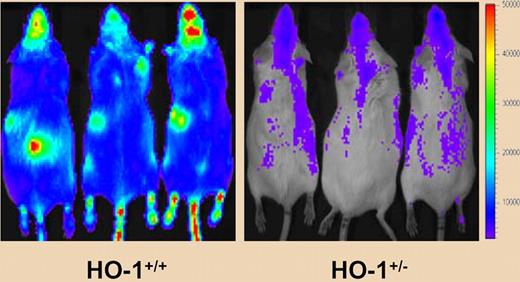
Ineffectiveness of primitive HO-1+/− bone marrow cells. In vivo bioluminescence imaging shows the reduced capacity of luciferase expressing bone marrow from HO+/− mice to reconstitute hematopoietic cells in lethally irradiated recipients. See the complete figure in the article beginning on page 4494.
Ineffectiveness of primitive HO-1+/− bone marrow cells. In vivo bioluminescence imaging shows the reduced capacity of luciferase expressing bone marrow from HO+/− mice to reconstitute hematopoietic cells in lethally irradiated recipients. See the complete figure in the article beginning on page 4494.
Studies over the last 2 decades have shown that the heme-oxygenases have an important protective role in many aspects of normal physiology. This is revealed in HO-1–deficient mice (HO-1−/−), which show a high level of embryonic lethality and anemia,2 and in a child with HO-1 deficiency, who had severe growth retardation, hemolytic anemia, coagulopathy, and early atherosclerosis.3 In comparison, HO-2–deficient mice survive longer and breed normally but experience long-term effects of chronic hypoxia.4
Cao et al now report that hematopoietic stem cells (HSCs) or progenitor cells from heterozygous HO-1–deficient mice (HO-1+/−) show increased proliferation and recovery of hematopoietic lineages after stress due to 5-FU treatment, transplantation, or a combination of phlebotomy and heme challenge. However, HO-1+/− HSCs have a reduced capacity to rescue lethally irradiated mice or to serially repopulate irradiated recipients. This suggests that HO-1 normally limits the proliferation and differentiation of hematopoietic progenitors during stress and that the failure of this mechanism can lead to premature exhaustion of the HSC pool. It will be interesting to discover if similar effects occur during aging.
A similar proliferative exhaustion of stem cells has been previously reported for mice deficient in Lig4,5 p21,6 and Gfi-1,7 when proliferation occurs in response to DNA damage or dysregulation of cell cycle control. Reduced HO-1–dependent breakdown of heme, and the absence of the antioxidant, antiproliferative and antiapoptotic effects of its metabolites might therefore affect HSC function in similar ways. In this case, however, the authors suggest that the most likely cause of increased proliferation during stress is reduced CO-dependent activation of p38MAPK pathway, leading to low levels of p21 in the rapidly dividing cells. They suggest that loss of one allele of HO-1 may be sufficient to maintain the steady-state metabolism of heme but insufficient under conditions of stress. The findings imply that HO-1 may play an important part in an evolutionarily conserved mechanism that balances proliferative capacity, stem cell function, and the response to environmental stress.
Another interesting implication of these results in HO-1+/− mice is that a similar and small quantitative difference in HO-1 gene expression during stress may have profound effects on cell function in humans. The human HO-1 gene promoter is characterized by a (GT)n repeat with common length polymorphisms affecting gene expression that have been associated with susceptibility to a wide variety of diseases.8 Thus, long GT repeat polymorphisms that cause lower expression of HO-1 are associated with emphysema, atherosclerosis, and stroke while shorter repeats are associated with increased susceptibility to cerebral malaria, some cancers, and miscarriages but have better liver and kidney transplant survival. The findings of Cao et al indicate that it will now be important to discover if and when these polymorphisms affect stem cell function in solid organs or in bone marrow, for example, after irradiation. The fact that several commonly used drugs (aspirin, statins, rapamycin and cyclosporine) induce or repress HO-1 heightens the importance of these results.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal