Abstract
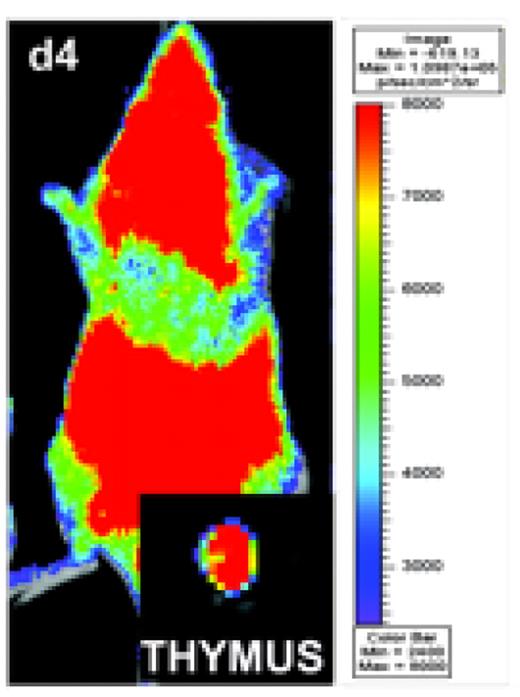
Although thymic graft-versus-host-disease (tGVHD) has been recognized as an important contributor to impaired T cell reconstitution, limited T cell repertoire and increased infection risk in patients with GVHD, the molecular basis of interactions between donor alloreactive T cells, donor bone marrow (BM)-derived thymocytes, and host hematopoietic and non-hematopoietic thymic stromal cells in GVHD has not been well-defined. Here we analyzed the role of molecules relevant for T cell trafficking, cytolytic function, and co-stimulation and co-inhibition of alloreactive T cells in tGVHD. We first demonstrated that thymic output (as measured by RAG2+ splenic recent thymic emigrants) as well as the thymic cellularity (especially of CD4+CD8+ thymocytes) were inversely proportional to numbers of mature donor T cells infused with the allograft, suggesting that tGVHD severity was inversely associated with thymic function. We then studied the migration of alloreactive donor T cells in vivo with bioluminescence imaging (BLI) and found that luciferase-expressing donor T cells infiltrated the thymus within one week after allogeneic bone marrow transplantation (BMT) (Fig. 1). Upon adoptive transfer of CFSE-labeled donor T cells we noted that thymus-infiltrating alloreactive donor T cells were largely fast-proliferating (CFSElo) and highly activated (CD25+ CD44+).
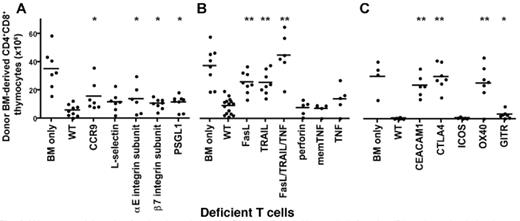
We analyzed the importance of T cell trafficking molecules for tGVHD using mice deficient for certain trafficking molecules, and assessed tGVHD by loss of BM-derived CD4+CD8+ thymocytes. We found that CCR9, b7 integrin subunit, and PSGL-1 were all partially required for tGVHD, while L-selectin and aE integrin subunit may be dispensable (Fig. 2A). Similarly, we examined the role of T cell cytolytic pathways for tGVHD, and found that FasL and TRAIL were required for tGVHD, but that perforin and TNF were dispensable (Fig. 2B). Finally, we assessed the role of various T cell co-stimulatory and co-inhibitory molecules for tGVHD, and found that CEACAM1, OX40 and CTLA4 were required, while GITR was partially required and ICOS was dispensable (Fig. 2C).
Upon further analysis of donor BM-derived thymocytes, we observed that Bcl-2 expression in donor BM-derived thymocytes was decreased in recipients with GVHD vs. those without GVHD, which suggests that survival of thymocytes is decreased during tGVHD. Hollander and others have previously demonstrated in non-irradiated GVH reaction models that host non-hematopoietic thymic stroma may be an important target for donor alloreactive T cells. We assessed the expression of the death receptors Fas and DR5 in thymic stroma from normal and irradiated (850 cGy) BALB/c mice. We observed that in particular, MHC class II-negative stroma (endothelial cells and fibroblasts), as well as a population of MHC class II-intermediate stroma (epithelial cells) upregulated the expression of both Fas and DR5 after irradiation.
Our study defines the specific pathways for cytolysis, trafficking and immune modulation involved in tGVHD and suggests selective therapeutic targets to attenuate tGVHD and improve post-transplant T-cell reconstitution in patients with GVHD.
BLI demonstrate a distinct distribution pattern for alloreactive donor T cells in allogeneic BMT recipients, Allogeneic Balb/c recipients show a strong signal on day 4 post-transparent after transfer of 10×108 luc+ splenocytes as measured by total body photon emission. Ex vivo imaging confirms the infiltration of luc+ splenocytes to the thymus.
BLI demonstrate a distinct distribution pattern for alloreactive donor T cells in allogeneic BMT recipients, Allogeneic Balb/c recipients show a strong signal on day 4 post-transparent after transfer of 10×108 luc+ splenocytes as measured by total body photon emission. Ex vivo imaging confirms the infiltration of luc+ splenocytes to the thymus.
We assessed the role of molecules relevant for T cell trafficking (A), cytolytic function (B), and co-stimulation, co-inhibition (C). Irradiated BALB/c mice received 5×106 T cell depleted C57BL/6 bone marrow + 0.25×106 purified splenic T cells. Absolute numbers of donor-BM-derived CD4+CD8+ thymocytes are shown. Black bars indicate means.
p-values were calculated vs. recipients of WT T cells(*p<0.05, **p<0.01)
We assessed the role of molecules relevant for T cell trafficking (A), cytolytic function (B), and co-stimulation, co-inhibition (C). Irradiated BALB/c mice received 5×106 T cell depleted C57BL/6 bone marrow + 0.25×106 purified splenic T cells. Absolute numbers of donor-BM-derived CD4+CD8+ thymocytes are shown. Black bars indicate means.
p-values were calculated vs. recipients of WT T cells(*p<0.05, **p<0.01)
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal