Abstract
Background: Pediatric thalassemia major (TM) patients may begin lifelong iron chelation therapy (ICT) as early as 2 years to avoid potential complications of iron overload, such as impaired growth and the later development of cardiac dysfunction. Deferasirox (Exjade®), administered orally once daily, was developed to improve compliance to long-term ICT in patients with transfusion-dependent anemias. We report cumulative efficacy, safety and development data in pediatric patients with transfusion-dependent TM treated with deferasirox for 5 years (study 106).
Methods: Pediatric TM patients stratified into two age groups (children 2-≤12 years; adolescents 12–17 years) were enrolled in study 106 and received deferasirox 10 mg/kg/day for 1 year. Patients completing the 1 year study were able to continue deferasirox treatment for an additional 4 years at adjusted doses, to evaluate long term safety and effect on liver iron concentration (LIC). Safety was assessed by the incidence and type of adverse events (AEs), laboratory parameters and growth/sexual development. Efficacy was evaluated by changes in LIC by SQUID.
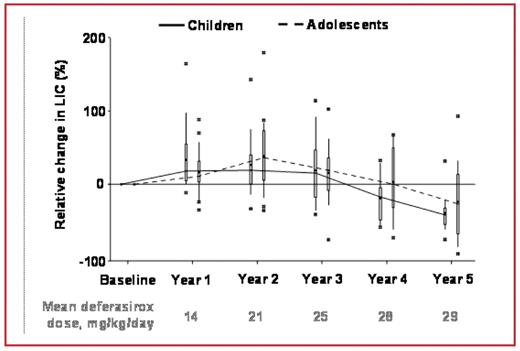
Results: 20 children (mean age 6.7±2.8 years) and 20 adolescents (mean age 14.1±1.6 years) were enrolled. Children had a greater mean iron intake (0.46–0.49 mg/kg/day) than adolescents (0.39–0.41 mg/kg/day). Median exposure to deferasirox was 5.0 years for children, 5.5 years for adolescents; mean dose in children and adolescents was 18.9±5.7 and 20.9±4.5 mg/kg/day, respectively. On average, 30% of children and 10% of adolescents received <15 mg/kg/day. Mean final dose was 26.3 mg/kg/day for children and 27.8 mg/kg/day for adolescents. Relative change in LIC over the treatment period is shown in Figure 1.
Boxplot of relative change in LIC during deferasirox treatment, by age group
Boxplot of relative change in LIC during deferasirox treatment, by age group
Overall, 24 patients (60.0%; 11 children and 13 adolescents) completed the 5-year study. Reasons for discontinuation were AEs (n=8), consent withdrawal (n=7), unsatisfactory therapeutic effect (n=1). Most common AEs were cough, pyrexia (n=34, 85%) and rhinitis (n=30, 75%); the annual frequency of reported AEs did not increase from year to year and generally occurred in similar proportions for children and adolescents. One child had a serious AE (increased transaminases) assessed as drug-related. Two other patients had an ALT increase >10 × ULN on at least one visit; baseline levels were >8 × ULN in one patient, and normal in the other. Both patients were restarted on deferasirox after 2 week interruptions without further incident. No patient had an increase in serum creatinine >33% above baseline and ULN at two consecutive visits. Neutropenia (neutrophil count <1.5×109/L at two consecutive visits), assessed unrelated to treatment, occurred in one child and two adolescents. There were no clinically significant visual defects and one child had a drug-related audiometric abnormality (hypoacusis). Physical development was normal in both groups; growth velocity was higher in children. Sexual development progressed normally in adolescents.
Conclusions: Deferasirox dose-dependently reduced iron overload over 5 years of treatment in these heavily transfused pediatric patients with TM. Dose increases over time, with a stable iron intake, were associated with a decrease in LIC for both groups, but the reduction in LIC was greater in adolescents. Despite having a higher transfusional iron intake, more children than adolescents were on a dose <15 mg/kg/day, highlighting that transfusional iron intake should be considered when selecting deferasirox dose. Deferasirox was generally well tolerated. There was no evidence of progressive renal, hepatic or bone marrow dysfunction. Deferasirox treatment had no negative impact on growth and sexual development.
Disclosures: Piga:Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Apopharma: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Galanello:Novartis: Honoraria, Research Funding. Jehl:Novartis: Employment. Rebischung:Novartis: Employment, Equity Ownership. Maseruka:Novartis: Employment, Equity Ownership. Rojkjaer:Novartis: Employment. Forni:Novartis: Research Funding.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal