Abstract
Introduction. Platelets contain a mixture of growth factors (GF). These can be released from platelet lysate (PL) whose application to wounds is supposed to favour the healing process. The application of PL in a suitable formulation can improve therapeutic efficacy and patient compliance. A formulation of PL with a selected biocompatible vehicle has been developed for application in buccal mucosal damages (mucositis), that are the most common complications of the nonsurgical therapy of cancer. The same vehicle can be proposed also for ophthalmic formulations as several studies have shown that topical application of the growth factors to the injured cornea facilitates wound repair by rapid regrowth of the epithelial cells. Aim of the present work was to develop in vitro tests for a fast evaluation of PL activity in the formulation to evidence excipient compatibility, and the effect of formulative parameters on PL stability.
The proliferative effect on two different models, fibroblasts and a corneal epithelial cell line, was evaluated. The stability of PL in the formulation has been assessed after two weeks at 4–8 °C storage.
Methods. PL was mixed in a 1:1 ratio to the selected vehicle and stored at 4–8 °C. Immediately after preparation (time zero, T0) and after 2, 7, 10 and 15 days (T2–T15), aliquots were used to assess proliferative effect on cell cultures. Both fibroblast (primary human cell lines) and RCE (Rabbit corneal epithelial cell lines, ECACC) were seeded in 96-well plates with area of 0.34 cm2 at the concentrations of either 10000 or 5000 cells/well. After 24 hours the cells were added with either complete growth medium (Reference), minimal medium not supplemented with foetal calf serum and for REC cells without also EGF (Control), PL formulation diluted 1/20 (containing PL at 5%) and PL formulation diluted 1/40 (containing PL at 2.5%). After 24 hours, neutral red (NR) test was performed (Tox Kit 4, Sigma-Aldrich, Milano I). The NR solution absorbance was determined by means of a plate reader (Perkin Elmer, Milan, I) at wavelength of 490 nm. The absorbance read for each sample was compared with that of Reference, whose proliferation was assumed as 100%.
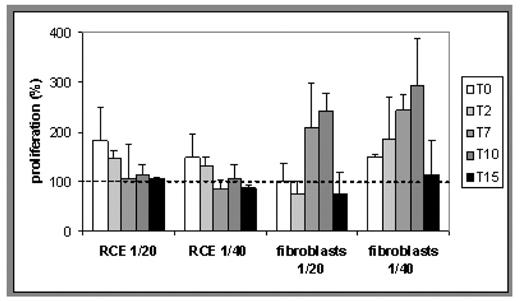
Results. At time zero, the percentage of proliferation showed no significant differences with respect to the cells in complete growth medium (Reference) in the case of fibroblasts, both at 5000 and 10000 seeding level. The two cell models gave comparable results although slightly higher values of proliferation were obtained in fibroblasts. This can be attributed to the epithelial nature of the RCE and to the possible different percentage in the platelet derived mixture of FGF and EGF. In both cases it can be argued that the vehicle is compatible with cell growth. Both for fibroblasts and for RCE no decrease of activity with time was observed, and no statistical differences with respect to the control have been obtained until 15 days storage. (See figure 1).
The results obtained suggest that the two cell culture models evaluated can be suitable to assess the activity of PL in easy and fast way. This will be useful to develop formulations intended for the treatment of mucositis and of damages of corneal epithelia. In particular, the formulation tested seems to be stable and to allow the release of GF from PL until 15 days of storage.
Proliferation (5000 cells/well) induced by the PL formulation as a function of storage time. The dotted line indicates the proliferation of the Reference (assumed as 100%).
Proliferation (5000 cells/well) induced by the PL formulation as a function of storage time. The dotted line indicates the proliferation of the Reference (assumed as 100%).
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal