Abstract
Background: As pediatric patients with β-thalassemia will require lifelong iron chelation therapy, it is important to evaluate the long-term efficacy, safety and growth during treatment with any iron chelator. This analysis presents cumulative efficacy and safety data from a cohort of pediatric patients treated with the once-daily, oral chelator deferasirox during two 1-year core and 4-year extension trials.
Methods: β-thalassemia patients aged 2–<16 years with transfusional iron overload were enrolled in two extension studies (107E and 108E) evaluating the long-term safety, tolerability and efficacy (by serum ferritin [SF] levels) of deferasirox; only patients who received deferasirox from the beginning of the core trial are included in this analysis. Deferasirox doses in the extension trials were initially based on end-of-core liver iron concentration, and were adjusted according to trends in SF levels. Efficacy was monitored by monthly SF levels; safety was assessed by the incidence and type of adverse events (AEs) and laboratory parameters.
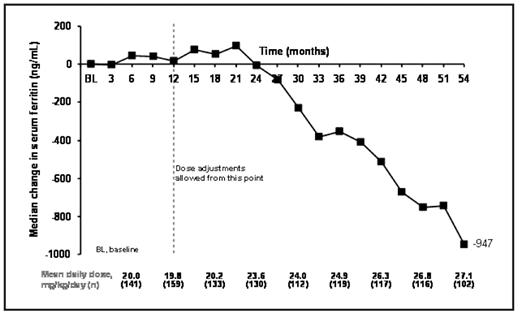
Results: 168 patients (153 patients from study 107 and 15 from study 108) aged 2–<6 (n=30), 6–<12 (n=74) and ≥12–<16 (n=64) years entered the extensions. To date, patients have received deferasirox for a median of 56.1 months (4.7 years) at an average daily dose of 22.5 ± 6.6 mg/kg/day. Mean iron intake during treatment was 0.4 ± 0.1 mg/kg/day. Mean actual dose at month 3 was 19.8 ± 8.8 mg/kg/day, which increased to 28.3 ± 7.3 mg/kg/day by month 54. Median SF levels at baseline were 2419 ng/mL and were maintained to around month 18, reflecting the mean dose of ~20 mg/kg/day (Figure 1). Following dose titration, SF levels began to decrease with an overall median decrease in SF by month 54 of −947 ng/mL.
At the time of analysis 137 patients (81.5%) continue to receive deferasirox. Of 31 discontinuations, 13 (7.7%) were due to AEs (drug-related AEs leading to discontinuation included glycosuria [n=3] and proteinuria [n=2]), eight (4.8%) to unsatisfactory therapeutic effect, five (3.0%) to consent withdrawal and four (2.4%) to other reasons. One death (septicemia in a splenectomized patient) occurred, considered by the Program Safety Board unrelated to treatment. Over the study period, the most common drug-related (investigator-assessed) AEs were abdominal pain and vomiting (n=12, 7.1% for both), nausea (n=10, 6.0%) and rash (n=9, 5.4%). The annual frequency of drug-related AEs decreased from year to year. In total, 13 patients (7.7%) had an increase in serum creatinine >33% above baseline and the upper limit of normal (ULN) on two consecutive visits; however, there were no progressive increases. Six patients (3.6%) with a normal baseline alanine aminotransferase (ALT), and five (3.0%) with a baseline ALT >ULN had an ALT increase >10×ULN on at least one visit. Growth, as assessed by height, proceeded normally in this population.
Conclusions: Over a median period of 4.7 years, deferasirox treatment provided a dose-dependent overall reduction in iron burden in transfusion-dependent children with β-thalassemia, as measured by SF levels. Deferasirox was generally well tolerated with the frequency of investigator-reported AEs decreasing over long-term treatment.
Mean dose and median change in SF during deferasirox treatment in pediatric β-thalassemia patients
Mean dose and median change in SF during deferasirox treatment in pediatric β-thalassemia patients
Disclosures: Piga:Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Apopharma: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Forni:Novartis: Research Funding. Kattamis:Novartis: Consultancy, Honoraria, Speakers Bureau. Aydinok:Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau; Apotex: Honoraria, Membership on an entity’s Board of Directors or advisory committees. Rodriguez:Novartis: Employment. Rojkjaer:Novartis: Employment. Galanello:Novartis: Honoraria, Research Funding.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal