Abstract
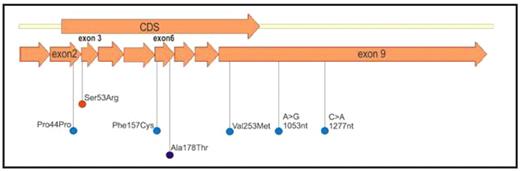
Congenital methemoglobinemia due to cytochrome b5 reductase (CYB5R3) deficiency are sporadic worldwide, although clusters of this disorder have been identified in certain ethnic groups. Here we describe the molecular basis of type I methemoglobinemia due to CYB5R3 deficiency in four patients representing three distinct ethnic backgrounds. Three subjects were of Indian ethnicity one being phenotypically normal and his two cousins diagnosed with type I methemoglobinemia. Causative mutations in the globin genes were excluded after sequencing. A fourth subject, a 12 year old Mexican child with Turner’s syndrome and apparent congenital cyanosis was referred to us for evaluation. Her methemoglobin level was elevated and erythrocyte cytochrome b5 reductase was reduced (4% of the normal range). The fifth individual, a 36 year old woman of Greek ancestry, had a past history of childhood cyanosis and low O2 saturation (82%) post-surgery in 2007. In early 2008 she was diagnosed with methemoglobinemia due to dyspnea, disproportionate fatigue, and significant increase in methemoglobin levels (10%). Genomic DNA from all 5 subjects was isolated, and all nine exons of the CYB5R3 gene were PCR amplified and sequenced. A non-synonymous heterozygous mutation (G>A transversion) in exon 6 resulting in the change of amino acid 178 from alanine to threonine (A178T) was identified in both affected Indian siblings and not in their unaffected cousin. This mutation which is located in the NADH binding domain and in close proximity to the adenosine moiety, and may influence binding of the NADH coenzyme had been previously reported (Dekker et al., 2001). A novel homozygous mutation in an evolutionarily conserved position located in exon 3 (C>G transition) resulting in substitution of serine 53 for arginine (S53R) was found in the child of Mexican descent. This mutation, located within the FAD binding domain, is hypothesized to cause disruption of hydrogen bonding and steric hindrance due to the bulkier arginine side chain, consequently leading to protein instability. Five heterozygous mutations were detected in the subject with Greek ancestry. A synonymous G>A substitution (Pro44Pro) in exon 2, a previously reported type II mutation G>A (Val253Met) and two 3′-UTR single nucleotide polymorphisms (A>G at 29690 nt, and C>A at 29914 nt), all in exon 9, were found in a cis configuration. A novel heterozygous compound mutation in exon 6, a T>G transversion, resulting in substitution of phenylalanine 157 for cysteine (F>C) was identified in the same subject with Greek ancestry (Figure 1). PCR amplified CYB5R3 transcripts harboring either wild-type, S53R, or F157C sequences were cloned, in frame with the 10X histidine tag, into the pET16b expression vector. Sequence fidelity and in frame fusion of all constructs was verified by sequencing from both strands. Recombinant protein was purified using 1 mL niquel-affinity columns. Kinetic studies using recombinant CyB5R3 enzyme will follow.
Location of mutation found in 4 cases of congenital type I methemoglobinemia.
Location of mutation found in 4 cases of congenital type I methemoglobinemia.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal