Abstract
Advanced myelodysplastic syndrome and secondary or relapsed AML are difficult to treat, with CR rates following standard induction chemotherapy less favorable than those for de novo AML. We examined an induction approach using the anti-CD33-calicheamicin conjugate gemtuzumab ozogamicin (GO) in combination with conventional chemotherapy.
Methods and Patient Population: We present data from a completed phase I/II study, demonstrating the activity and tolerability of the combination in patients with MDS and AML. 44 pts (19 pts from phase I, 25 from phase II) were assigned to receive cytarabine 2 g/m2 over 4 hours once daily, and topotecan 1.5 mg/m2 by continuous infusion daily on days 1–5. GO was given on day 6 at a dose of 3 mg/m2 (7 pts), 4.5 mg/m2 (28 pts), or 6 mg/m2 (7 pts); 2 additional pts did not receive the GO because of intercurrent toxicities. The MTD of GO was established at 4.5 mg/m2, which was used in phase II. The study population consisted of 15 pts with advanced MDS (IPSS 2–3), 23 pts with previously untreated secondary AML, and 6 pts with relapsed AML. Median age was 64 (34–77); 34 of 44 (77%) pts were over age 60.
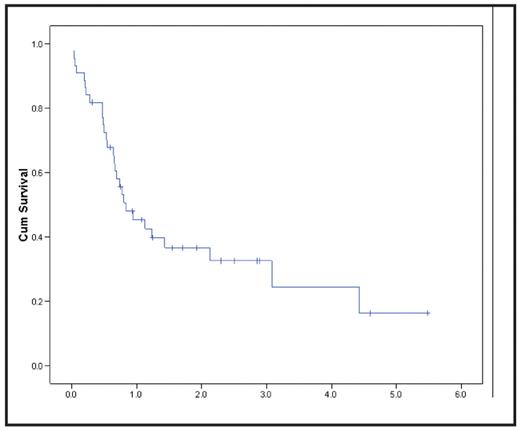
Results:ATGO was generally well tolerated. Infections, including 15 bacteremic episodes, were the most common non-hematologic complications. Two cases of reversible but unexplained grade 3 delirium were seen at 6 mg/m2 GO, meeting study criteria for dose-limiting toxicity. Only one case (3%) of clinical venoocclusive disease (VOD/SOS) occurred, in the single subject who received a second (half) dose of GO on day 16. Reversible and asymptomatic grade 3 hepatic transaminase elevations occurred in 8 pts (18%). There were 4 deaths (9%) prior to day 40: 2 infectious, 1 CNS bleed, and 1 sudden death. Based on intent to treat, 25 pts (56.8%) achieved complete morphologic remission, with or without full platelet recovery after one cycle of induction. The CR/CRp rate for pts over age 60 was similar to that for younger pts (59% vs 53%, respectively, p=NS). Similar proportions of pts with MDS, secondary AML, and relapsed AML achieved CR/CRp (60% vs 52% vs 67%, respectively, p=NS). Following ATGO induction, the majority of responders went on to receive post-remission therapy, including 11 pts who underwent allogeneic hematopoietic cell transplantation (HCT). Post-remission therapies, including HCT, were not associated with VOD/SOS or unusual toxicities. Median survival for all patients was 303 d (95% CI, 138–467 d), and the K-M estimated 2 year survival is 37%. As expected, achievement of CR after ATGO was associated with improved overall survival, and there was a trend toward improved survival in younger pts. Cytogenetic class, type of disease (MDS v. AML), and administration of prior chemotherapy were not significant predictors of either CR or OS. Ten pts (23%) are alive, with a median follow-up of 768 days (275–2001); 7 (16%) are currently in continuous CR, including 4 of the 11 pts who received allogeneic HCT.
Conclusions: Our data show that ATGO is a well tolerated induction regimen, and is not associated with immediate or late VOD after a single dose of GO. The CR rate with this anthracycline-free induction regimen is promising across a range of ages and myeloid disease categories. Furthermore, the tolerability of ATGO is such that effective post-remission therapy including allogeneic HCT is feasible in responding pts.
Overall Survival Years
Disclosures: Langston:Galxo-Smith Kline: Research Funding; Wyeth: Research Funding. Off Label Use: Topotecan is not FDA approved for myeloid malignancies. Gemtuzumab ozogamicin is approved for use in relapsed AML only. This trial qualifies for exemption from need for IND, and is IRB approved. Conduct of the trial was supported by Wyeth and Glaxo-Smith-Kline.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal