Abstract
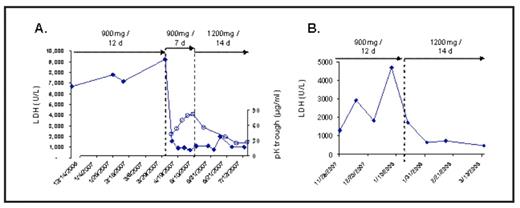
Paroxysmal nocturnal hemoglobinuria (PNH) is characterized by a lack of the terminal complement inhibitor CD59 on erythrocytes that renders these cells susceptible to chronic hemolysis. Eculizumab blocks terminal complement resulting in reductions in hemolysis, thrombotic events, renal impairment and transfusion requirement, as well as improvement in quality of life. The standard dosing regimen for eculizumab is 600 mg/week for 4 weeks (induction); 900 mg one week later; and then 900 mg every 14 ± 2 days (maintenance). This regimen maintains eculizumab levels >35 μg/mL, which is sufficient to completely and consistently block complement-mediated hemolysis in patients with PNH. In PNH clinical trials, 900 mg of eculizumab every 14 ± 2 days effectively and consistently blocked complement-mediated hemolysis in 98% of patients (n=195). During the studies, 10–15% of patients experienced an increase in hemolysis (elevation of LDH) near the end of the 14-day dosing interval with a return of pre-eculizumab symptoms such as hemoglobinuria, dysphagia, abdominal pain, or fatigue. The dosing interval was reduced to 12 days, as specified by label, resulting in sustained complement blockade, control of hemolysis and resolution of symptoms in nearly all patients. Three of the original 195 patients (2%) were not consistently blocked with the approved dosing regimen. Alternative eculizumab dosing regimens were investigated in these patients to assess their effectiveness and safety. Two different dosing regimens were employed; both included a maintenance phase with 1200 mg every 14 days. One regimen also included an induction period of 900 mg weekly for 5 doses. LDH, pharmacokinetics (PK), and clinical signs of complement breakthrough were monitored. The time from first eculizumab treatment to initial breakthrough on the 900 mg every 14 days ranged from 2 to 19 mo., and the reduction in the dosing interval to 900 mg every 12 days, as specified in the label, did not adequately control hemolysis in each of these 4 patients. Patient 1 was managed for 6 mo. with 900 mg every 12 days before experiencing additional complement breakthrough episodes (Figure, panel A). LDH levels (closed diamonds) reached 9234 U/L (ULN, 430-450 U/L) and breakthrough symptoms occurred 2 days prior to the next dose. The patient was re-induced with 900 mg eculizumab every 7 days for 5 weeks followed by 1200 mg every 14 days. Trough levels of eculizumab increased (open circles) each week during the induction phase (42.7 – 81.8 μg/ml) resulting in an immediate reduction in LDH to near normal levels. A maintenance dose of 1200 mg every 14 days in this patient resulted in sustained complement blockade. Patient 2 experienced breakthrough hemolysis after 19 mo. of standard dosing. Complement breakthrough occurred during a post-cholecystectomy infective endocarditis. After an adjustment to 900 mg every 12 days did not control complement breakthrough (Figure, panel B), the dose was changed to 1200 mg every 14 days without re-induction. This regimen resulted in sufficient levels of eculizumab to consistently reduce hemolysis to near normal levels. Further episodes of hemoglobinuria and other symptoms of hemolysis were not observed. Two additional patients received 1200 mg every 14 days without re-induction, one following complement breakthrough on the approved dose and the other due to the convenience of the 14 day interval with the 1200 mg dose. Complete complement blockade has been maintained in these patients for 8 mo. and 12 mo. to date, respectively. After 1 year of sustained complement blockade with the 1200 mg maintenance dose, patient 1 again demonstrated a breakthrough. Complement inhibition is now being maintained in this patient by a 1200 mg dose every 14 days with an additional 1200 mg dose in between the 14 day dosing interval every 4–5 doses. There were no reported adverse events in any of the four patients in which the 1200 mg dosing regimens were administered. In summary, these data demonstrate good correlation between eculizumab and LDH levels, suggesting that a breakthrough in complement activity due to insufficient drug levels can be monitored by levels of LDH near the end of the dosing interval. These results illustrate that two alternative-dosing regimens are well tolerated and can be effectively employed in the small percentage of PNH patients in which complement inhibition is not consistently maintained using the standard dose.
Disclosures: Kelly:Alexion Pharmaceuticals: Membership on an entity’s Board of Directors or advisory committees, Research Funding. Richards:Alexion Pharmaceuticals: Consultancy, Membership on an entity’s Board of Directors or advisory committees. Hill:Alexion: Honoraria, On scientific advisory board. vanBijnen:Alexion Pharmaceuticals: Research Funding. Muus:Alexion Pharmaceuticals: Membership on an entity’s Board of Directors or advisory committees, Research Funding. Brodsky:Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees. Khursigara:Alexion Pharmaceuticals: Employment, Equity Ownership. Rother, PhD:Alexion Pharmaceuticals: Employment, Equity Ownership, Patents & Royalties. Hillmen:Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal