Abstract
INTRODUCTION: Eltrombopag (PROMACTA®/REVOLADE®; GlaxoSmithKline, Collegeville, PA) is the first oral, small molecule, non-peptide thrombopoietin receptor agonist under investigation for the treatment of thrombocytopenia due to various causes, including idiopathic thrombocytopenic purpura (ITP). Chronic ITP is characterized by autoantibody-induced platelet destruction and reduced platelet production, leading to chronically low peripheral platelet counts. Eltrombopag treatment has previously demonstrated a significant increase in platelet counts and a reduction in clinically relevant bleeding symptoms in 2 placebo-controlled trials evaluating a total of >200 patients with chronic ITP after up to 6 weeks of treatment. EXTEND is an ongoing open-label, phase III extension study to assess the long-term safety and efficacy of oral eltrombopag in ITP patients that have previously completed an eltrombopag trial.
METHODS: Patients with previously treated, chronic ITP who completed a prior eltrombopag study were eligible to participate in EXTEND. Eltrombopag treatment was initiated at 50 mg once daily and then adjusted in order to maintain platelet counts ≥ 50,000/μL and <400,000/μL, with doses between 75 mg once daily and 25 mg once daily or less often than once daily, if necessary. Patients who achieved platelet counts ≥ 50,000/μL during treatment with eltrombopag were considered responders. Bleeding events were prospectively evaluated using the WHO Bleeding Scale: Grade 0 = no bleeding, Grade 1 = mild bleeding, Grade 2 = moderate bleeding, Grade 3 = gross bleeding and Grade 4 = debilitating blood loss.
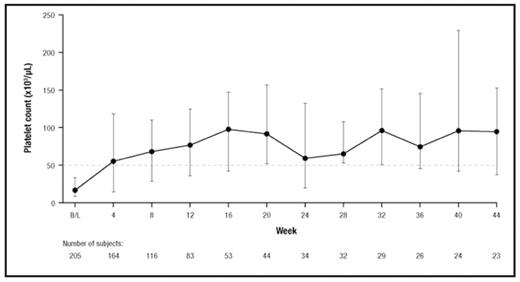
RESULTS: At the time of this analysis, 207 patients (median age, 50 years; 67% female) had received eltrombopag on this study. At baseline, 33% were receiving concomitant ITP medication and 40% were splenectomized. The majority of patients (70%) enrolled with baseline platelet counts <30,000/μL, followed by 18% and 12% with baseline platelet counts from ≥ 30,000/μL to ≤ 50,000/μL, and >50,000/μL, respectively. The duration of eltrombopag treatment ranged from 3 to 523 days. Seventy-nine percent (159/201) of patients achieved a platelet count ≥ 50,000/μL, and 24% (18/75) of patients who had received eltrombopag for at least 25 weeks maintained platelet counts ≥ 50,000/μL continuously for ≥ 25 weeks. Patients responded to eltrombopag regardless of splenectomy status (non-splenectomized: 78%, splenectomized: 81%) and use of baseline concomitant ITP medications (no baseline ITP medications: 79%, baseline ITP medications: 80%). Median platelet counts remained ≥ 50,000/μL throughout the observation period of the study (Figure 1) with only 3 exceptions, when the median platelet counts remained >40,000/μL. At baseline, 59% of patients reported bleeding symptoms (WHO Grades 1–4) compared with approximately 30% at months 1, 3, and 6. Adverse events (AEs) were reported in 150 patients (72%) while on therapy, the majority of which were mild to moderate. Headache (15%) was the most commonly reported on-therapy AE, followed by upper respiratory tract infection (13%), diarrhea (10%), and nasopharyngitis (9%). Six thromboembolic events were reported during the study. No clinically relevant effects of eltrombopag on patient bone marrow were detected. Thirty-nine serious AEs were reported by 17 patients (8%) while on therapy +1 day. Four deaths were reported in the study (2 deaths on therapy and 2 deaths >30 days after the last dose of eltrombopag); none were considered related to study medication.
CONCLUSION: Oral eltrombopag is effective at raising platelet counts and decreasing bleeding symptoms during long-term treatment, regardless of splenectomy status or the use of baseline ITP medications. Eltrombopag is well tolerated during long-term treatment in patients with previously treated chronic ITP.
Median platelet counts.a BL, median baseline value. aDotted line indicates 50,000 platelets/μL.
Median platelet counts.a BL, median baseline value. aDotted line indicates 50,000 platelets/μL.
Disclosures: Bussel:GlaxoSmithKline: Equity Ownership, Honoraria, Research Funding. Cheng:GlaxoSmithKline: Consultancy, Honoraria. Bailey:GlaxoSmithKline: Employment. Stone:GlaxoSmithKline: Employment. Aivado:GlaxoSmithKline: Employment, Equity Ownership.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal