Abstract
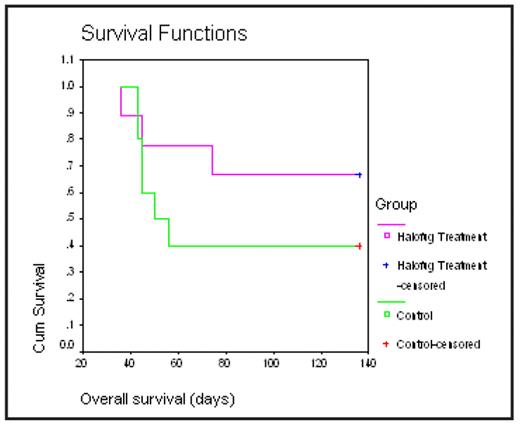
Halofuginone, a synthetic derivative of quinazolinone alkaloid, previously has been shown to have anti-cancer effects in various solid and hematological malignancies. Halofuginone inhibits mainly collagen type I synthesis, and extracellular matrix formation, via the inhibition of TGFβ signaling, matrix metalloproteinase 2(MMP2), and angiogenesis. Last year, we first reported, that Halofuginone in a low doses (IC50 of 50—100 nM) induces cytotoxicity in multiple MM cell lines, including cells resistant to conventional (e.g., dexamethasone, alkylating agents, and anthracyclines) or novel (e.g. thalidomide and bortezomib) anti-MM agents and overcomes the survival and growth advantages conferred by interleukin-6, insulin-like growth factor-1 and by bone marrow stroma cells. Halofuginone induced apoptosis in a caspase 3, 8, and 9 dependent mechanisms, reduced mitochondrial membrane potential, and down regulated MCL1 protein. We now assessed the cytotoxic effect of Halofuginone in primary MM patient cells in vitro and, its effect on tumor growth and survival in in vivo models. We found that Halofuginone also induces growth inhibition and cell death in primary MM cells (n=4, IC50: 100–200nM). Importantly, Halofuginone demonstrated additive or synergistic effects with some of the established anti-MM agents such as Melphalan, Dexamethasone, and Lenalidomide. In addition, Halofuginone inhibits IL6 production in the supernatant of a co-culture of MM.1S cells with HS-5 stromal cell line. Mechanistically, Halofuginone induces MM cell death, which involves the up-regulation of c-jun NH2-terminal kinase signaling (JNK), c-Jun, as well as the p-53 proapoptotic protein. Additionally, the in vivo anti-MM activity of Halofuginone was evaluated in 2 separate in vivo models, a xenograft model in SCID mice (subcutaneous injection of MM1S cells), and a model of diffuse MM lesions in SCID-beige mice (generated by i.v. injections of OPM-2 cells). In both models, mice were first sublethally irradiated (200 rads), injected s.c or i.v., respectively, with 1×106 MM cells and then randomly assigned to receive, either treatment with 0.75mg/kg halofuginone (IP or by oral gavage, respectively; n=10) or vehicle only (n=10) on a cyclical schedule of 5 days-on/2 days-off. In both models, Halofuginone inhibited MM tumor growth and improved survival, 70% vs 40% at 140 days (NS) in the treated vs control group respectively (figure). Clinical evidence of adverse events (weight loss, vomiting) were not observed. Halofuginone may, thus represents a promising novel orally bioavailable anti-MM agent that needs further evaluation for possible clinical trials in MM.
Diffuse MM lesions in SCID-beige mice. Animals were sub lethally irradiated (200rads), injected i.v. with 1×106 OPM2 cells and then randomly assigned to receive, either halofuginone treatment (by oral gavage; 0.75mg/kg (n=10) or vehicle only (n=10) on a cyclical schedule of 5 days-on/2 days-off.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal