Abstract
Background: Low oxygen levels are a defining characteristic of solid tumors, but the role of hypoxia in leukemogenesis remains unclear. Recent reports indicate that the endosteum at the murine bone-bone marrow (BM) interface is hypoxic, and data in a rat model demonstrate that leukemic cells infiltrating bone marrow were markedly hypoxic compared with cells in the BM of healthy rats. Hypoxia triggers a complex cellular and systemic adaptation mediated primarily through transcription by hypoxia inducible factors (HIFs) including HIF-1a. Although hypoxia is the best-characterized mechanism of HIF activation in tumors, HIF activity also can be induced in tumor cells through activation of the PI3K/Akt-signaling pathway. In this study, we assessed AKT and HIF-1a expression in newly diagnosed precursor B-cell acute lymphoblastic leukemia (pre-B ALL) and correlated the results with overall and progression-free patient survival.
Methods: We analyzed expression of phosphorylated AKT (pAKT) and HIF-1a in leukemic cells by immunohistochemical methods using archival fixed, paraffin-embedded BM biopsy specimens of newly diagnosed pre-B ALL and antibodies specific for pAKT (Cell Signaling Technology, Beverly, MA) and HIF-1a (BD Biosciences, San Jose, CA). The initial observations were confirmed by a Reverse Phase Protein Array (RPPA) data set generated from protein lysates prepared from fresh blood and BM aspirate samples from patients with newly diagnosed pre-B ALL.
Results: There were 26 men and 27 women with a median age of 39 years (range, 17–77). The median follow-up was 17 months (range, 1–71). The median WBC was 5.7 × 109/L (range, 0.8–369 × 109/L), the median percentage of blasts in bone marrow was 88% (range, 21–97%). Conventional cytogenetic studies detected a normal karyotype in 13 patients and abnormal karyotype in 37 patients including the Philadelphia chromosome (Ph) in 15 patients; no analyzable metaphases were recovered in 5 patients. Fluorescence in situ hybridization for BCR/ABL rearrangement was performed in all patients and was positive in all 15 patients with Ph and in 1 patient with normal conventional cytogenetics. 50 patients received HYPER-CVAD therapy, 3 patients received augmented BFM therapy. 49 (92%) patients achieved complete remission with a median time to response of 3 weeks (range, 2–8 weeks), 12 of them relapsed. 17 patients died, including 6 patients in complete remission. 3 year overall survival was 56% (CI, 46–66%). HIF-1a expression was detected in 37 (70%) patients, including 10 patients with Ph-positive ALL. HIF-1a expression was associated with expression of pAKT (R=0.4479, p<0.01), and was not associated with age, sex, WBC, percentage of blasts in blood or BM, platelet count, serum levels of albumin, beta-2-microglobulin, bilirubin, creatinine, LDH, or presence of the Ph.
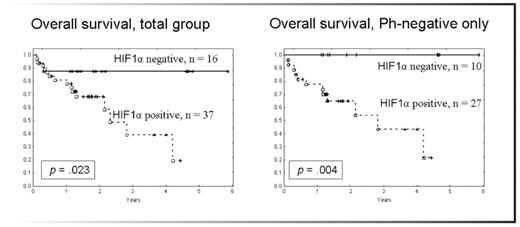
HIF-1a expression was associated with worse overall survival for the entire patient population (p=0.023). The negative prognostic impact of HIF-1a expression remained when only Ph-negative patients were analyzed (p=0.004), Fig 1. Patients with HIF-1a expression appear to have worse progression-free survival, but the difference did not reach statistical significance, p=0.39. These results were confirmed by RPPA data set generated from 104 patients with newly diagnosed Ph-negative ALL. HIF-1a overexpression was strongly associated with worse overall (p=0.026) and event-free (p=0.0178). No association of HIF-1a overexpression with other clinical or laboratory parameters was detected.
Conclusions: This is the first report demonstrating that HIF-1a expression is associated with worse overall and event-free survival in Ph-negative pre-B ALL. These findings implicate that inhibition of AKT signaling or blockade of HIF-1α-mediated pro-survival signaling events may improve clinical outcomes in pre-B ALL. Analysis of the key pro-survival signaling pathways activated by hypoxia and HIF-1a is ongoing (Frolova et al., ASH 2008).
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal