Abstract
Introduction: Megakaryocytic cells (Mks), the precursors to platelets, are among the least understood blood cell types. A primary aspect of Mk differentiation is endomitosis, whereby Mks duplicate their DNA content without undergoing cytokinesis and form cells with 4N, 8N, 16N, etc. Mk ploidy strongly correlates with platelet production. Thrombocytopenia accompanies several hematologic malignancies including myelodysplastic syndromes and is often associated with low in vivo Mk ploidy. Elucidation of the factors that regulate Mk endomitosis will aid in developing treatments for Mk-related disorders. We have previously shown that the B3 vitamin nicotinamide (NIC) causes a dose-dependent increase in Mk size and the fraction of high-ploidy (≥ 8N) Mks and leads to more complex proplatelet formation without affecting Mk commitment, ultrastructure, apoptosis, or viability in cultures of CD34+ cells (Giammona LM, et al. Br J Haem 135 (2006): 554). We examined whether NIC’s roles as an inhibitor of the sirtuin family of histone/protein deacetylases (SIRTs) and as a precursor for NAD+ were responsible for its effects on Mk ploidy.
Methods: CD34+ cells, isolated from healthy G-CSF-mobilized peripheral blood donors, were maintained in serum-free X-VIVO 20 media supplemented with 100 ng/mL thrombopoietin (Tpo). On day 5, cells were treated with 6.25 mM NIC, 10 μM cambinol (SIRT1/2 inhibitor), or 10 μM AGK2 (SIRT2 inhibitor) or maintained with Tpo alone. Flow cytometry was used to determine Mk commitment (CD41+), viability, apoptosis, ploidy, and intracellular levels of total and acetylated p53. The intracellular concentration of NAD(H) (NAD+ plus NADH) was determined using an enzymatic assay. Immunoblots were used to detect acetylated and total nucleosomes, as well as the NAD processing enzyme Nmnat1. p53 DNA-binding activity was determined using EMSA analysis.
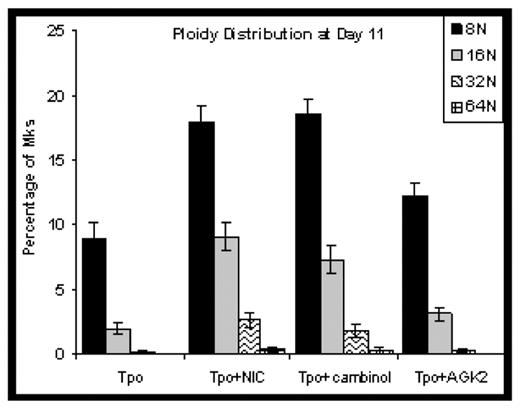
Results: Adding NIC to CD34+ cell cultures increased the percentage of high-ploidy Mks by 3-fold. The SIRT1/2 inhibitor cambinol increased Mk ploidy to a similar extent as NIC, while the SIRT2 inhibitor AGK2 was only 30% as effective. NIC and cambinol more than tripled the fractions of 16N and 32N Mks (Figure). None of the additives affected Mk commitment, viability, or apoptosis. Functional inhibition of SIRT1/2 by NIC was confirmed by increased acetylation of several SIRT1/2 target proteins. Both SIRTs deacetylate histones and we observed up to 3-fold greater nucleosome acetylation in cells treated with NIC. Flow cytometry showed that the ratio of AcK382p53 to total p53 was 3-fold higher in cells treated with NIC as compared to Tpo alone. Consistent with reports that acetylation increases p53 DNA-binding activity, EMSA analysis showed that p53 binding to the p53 consensus sequence was 50% greater in NIC-treated Mks. We have previously shown that p53 knockdown increases Mk ploidy in culture (Fuhrken PG, et al. J Biol Chem 283 (2008): 15589). These results suggest that increased p53 acetylation differentially affects different p53 target genes. NIC increased intracellular levels of NAD(H) by 5-fold. In contrast, an NAD+de novo pathway precursor had minimal impact on ploidy. NIC is incorporated into NAD+ via the salvage pathway, which is localized to the nucleus in yeast, whereas the de novo pathway is distributed throughout the cell. This suggests that NAD+ production in the nucleus may also play a role in NIC-mediated increases in Mk ploidy, and is consistent with higher nuclear levels of the NAD+ salvage pathway enzyme Nmnat1 detected in cells treated with NIC.
Conclusions: Inhibition of SIRT1 and SIRT2 appears to be the primary mechanism for NIC-mediated increases in Mk ploidy, and increased p53 acetylation is likely to play an important role in this process. Further study of SIRT targets associated with DNA repair, apoptosis, and cell cycle regulation may provide additional insight into Mk polyploidization.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal