Abstract
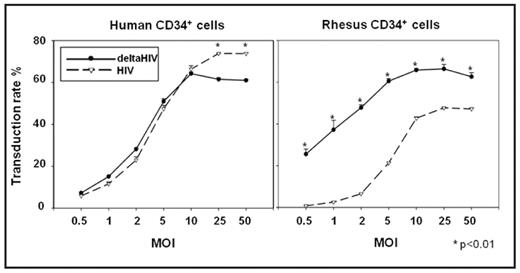
Although the successful application of gene transfer to hematopoietic stem cells using HIV based vectors for the hemoglobinopathies and thalassemias has recently been achieved in rodents, concerns regarding possible side effects, including insertional mutagenesis, mandate large animal preclinical testing. HIV vectors transduce rhesus blood cells poorly due to a species specific block by TRIM5a and APOBEC3G which target HIV capsid proteins (CAs) and viral infectivity factor (Vif), respectively. We developed a lentiviral vector capable of transducing both human and rhesus blood cells by combining components of both HIV and SIV by including SIV CA and SIV Vif. Titers of HIV vectors prepared with SIV Vif (8.4±0.69x106 IU/ml, p=0.73) or SIV CA (delta-HIV 8.1±1.1x106 IU/ml, p=0.73) were comparable to that of a normal HIV vector (9.2±0.46x106 IU/ml), while titers of chimeric HIV vectors that included SIV gag-pol or rev-tat were dramatically decreased (0.18-4.5x105 IU/ml, p<0.001). Unlike the HIV chimeric vectors, chimeric SIV vectors that included both HIV gag-pol and rev-tat showed relatively high titers when compared to normal SIV vectors (p=0.88), while titers of chimeric SIV vectors that included HIV CA were very low (p<0.01). To evaluate whether the candidate chimeric HIV vectors were able to transduce both human and rhesus blood cell lines, we performed dose-escalation transductions using the chimeric vectors. The delta-HIV vector showed superior transduction of human blood cell lines (61±2.8% at MOI=3, p<0.01) than the SIV vector (49±1.8%) and could efficiently transduce rhesus blood cell lines (18±0.90% at MOI=7, p<0.001) while the HIV vector could not (1.8±0.05%). To evaluate the transduction efficiency of delta-HIV on human and rhesus hematopoietic cells, we transduced human and rhesus CD34+ cells with delta-HIV and HIV vectors at various MOIs (0.5–50). In human CD34+ cells, the delta-HIV and HIV vectors showed similar transduction efficiency at lower MOIs (51±1.3%, 47±1.7% at MOI=5, p=0.18, respectively), while in rhesus cells, the transduction rates of delta-HIV (48±1.4% at MOI=2) were significantly superior to that of the HIV vector (6.5±0.36% at all MOIs, p<0.01). Among rhesus CFU, the delta-HIV vector produced a significantly higher percentage of GFP positive clones in comparison to those transduced with the HIV vector (Erythroid (E): 77±1.0% vs. 15±2.7% p<0.01, Myeloid (M): 46±1.8% vs. 1.2±0.59% p<0.01, respectively). On the other hand, among human CFU, similar rates of transgene expressing clones were observed (E: 79±0.79% vs. 72±0.64% p<0.01, M: 34±2.0% vs. 37±0.88% p=0.22, respectively). In erythroid and myeloid liquid cultures derived from transduced CD34+ cells, rhesus cells showed higher rates of transgene expression after delta-HIV transduction than after HIV transduction (E: 51±6.4% vs. 11±0.24% p<0.001, M: 27±2.5% vs. 6.1±2.0% p<0.001, respectively). In human erythroid and myeloid cells, the transgene expression rates were similar (E: 66±4.0% vs. 68±2.3% p=0.61, M: 36±1.5% vs. 37±1.5% p=0.93, respectively). In summary, we have developed an HIV-based lentiviral vector system capable of efficient transduction of both human and rhesus blood cells. This vector system should allow preclinical testing of HIV based therapeutic vectors in the large animal model.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal