Abstract
Introduction: We retrospectively analyzed outcomes of 716 patients with multiple myeloma who were mobilized using cyclophosphamide and growth factor (n=370) or growth factor alone (n=346) before stem cell transplantation.
Table. Patient Characteristics (N=716)
| Variable . | Cyclophosphamide (n=370) . | Growth Factor Only (n=346) . | PValue . |
|---|---|---|---|
| Abbreviation: IQR, interquartile range. | |||
| *Or equivalent dosage. | |||
| Men, No. of patients (%) | 224 (61) | 202 (58) | .50 |
| Age, median (IQR), y | 58 (52–64) | 60 (53–65) | .11 |
| b-2 Microglobulin, median (IQR), mcg/mL | 2.7 (1.9–4.0) | 2.3 (1.9–3.2) | .01 |
| Creatinine, median (IQR), mg/dL | 1.1 (0.9–1.3) | 1.0 (0.8–1.2) | .002 |
| Apheresis, median (IQR) collections, No. | 2 (1–3) | 4 (3–6) | .001 |
| Marrow plasma cells, % | 17 (5–34) | 5 (1–13) | .001 |
| CD34+cells, median (IQR), cells/kg | |||
| Total collected | 10.3x106(7.2x106–14.6x106) | 9.9x106(7.6x106–11.9x106) | .01 |
| Infused | 5.6x106(4.5x106–7.6x106) | 4.2x106(3.8x106–5.0x106) | <.001 |
| Duration of hospitalization, median (IQR), d | 4 (0–10) | 4 (0–9) | .92 |
| Nonstaphylococcal bacteremia, No. of patients (%) | 48 (13) | 25 (7) | .01 |
| Melphalan dosage, No. of patients (%) | .04 | ||
| 200 mg/m2* | 337 (91) | 298 (86) | |
| 140 mg/m2* | 33 (9) | 48 (14) | |
| Exposure before mobilization, No. of patients (%) | |||
| Melphalan | 45 (12) | 44 (13) | .50 |
| Lenalidomide | 14(4) | 63(18) | .01 |
| Variable . | Cyclophosphamide (n=370) . | Growth Factor Only (n=346) . | PValue . |
|---|---|---|---|
| Abbreviation: IQR, interquartile range. | |||
| *Or equivalent dosage. | |||
| Men, No. of patients (%) | 224 (61) | 202 (58) | .50 |
| Age, median (IQR), y | 58 (52–64) | 60 (53–65) | .11 |
| b-2 Microglobulin, median (IQR), mcg/mL | 2.7 (1.9–4.0) | 2.3 (1.9–3.2) | .01 |
| Creatinine, median (IQR), mg/dL | 1.1 (0.9–1.3) | 1.0 (0.8–1.2) | .002 |
| Apheresis, median (IQR) collections, No. | 2 (1–3) | 4 (3–6) | .001 |
| Marrow plasma cells, % | 17 (5–34) | 5 (1–13) | .001 |
| CD34+cells, median (IQR), cells/kg | |||
| Total collected | 10.3x106(7.2x106–14.6x106) | 9.9x106(7.6x106–11.9x106) | .01 |
| Infused | 5.6x106(4.5x106–7.6x106) | 4.2x106(3.8x106–5.0x106) | <.001 |
| Duration of hospitalization, median (IQR), d | 4 (0–10) | 4 (0–9) | .92 |
| Nonstaphylococcal bacteremia, No. of patients (%) | 48 (13) | 25 (7) | .01 |
| Melphalan dosage, No. of patients (%) | .04 | ||
| 200 mg/m2* | 337 (91) | 298 (86) | |
| 140 mg/m2* | 33 (9) | 48 (14) | |
| Exposure before mobilization, No. of patients (%) | |||
| Melphalan | 45 (12) | 44 (13) | .50 |
| Lenalidomide | 14(4) | 63(18) | .01 |
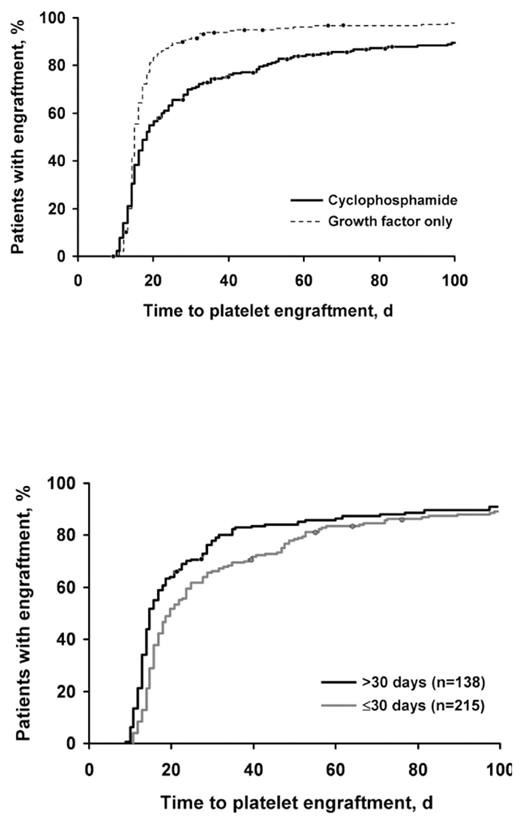
Results: Patients receiving cyclophosphamide had higher stem cell yields than the growth factor only group (median number of apheresis sessions needed to achieve stem cell collection goals, 2 vs 4 sessions, respectively [P=.001]). However, patients treated with cyclophosphamide required more time for engraftment of platelets and neutrophils (P<.001 for both).
For patients receiving cyclophosphamide, 75% achieved engraftment (defined as a platelet count of 50x109/L) by day 39, whereas 75% of patients not receiving cyclophosphamide achieved engraftment by day 18. Similar results were observed for neutrophil engraftment. These differences did not affect the duration of hospitalization, but patients treated with cyclophosphamide had a higher incidence of posttransplant nonstaphylococcal bacteremia. For cyclophosphamide-mobilized patients, considerably faster platelet engraftment (5 fewer days) resulted if stem cell reinfusion occurred more than 30 days after the first apheresis session.
Conclusion: Our data suggested that cyclophosphamide damaged the microenvironment and slowed engraftment. By lengthening the period between the completion of apheresis and stem cell reinfusion, the microenvironment may recover and result in faster engraftment. We suggest that the cyclophosphamide used for mobilization may affect the marrow microenvironment. Cyclophosphamide improves yields but increases bacteremia rates after transplantation. Cyclophosphamide increases time required to collect stem cells and slows engraftment.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal