Abstract
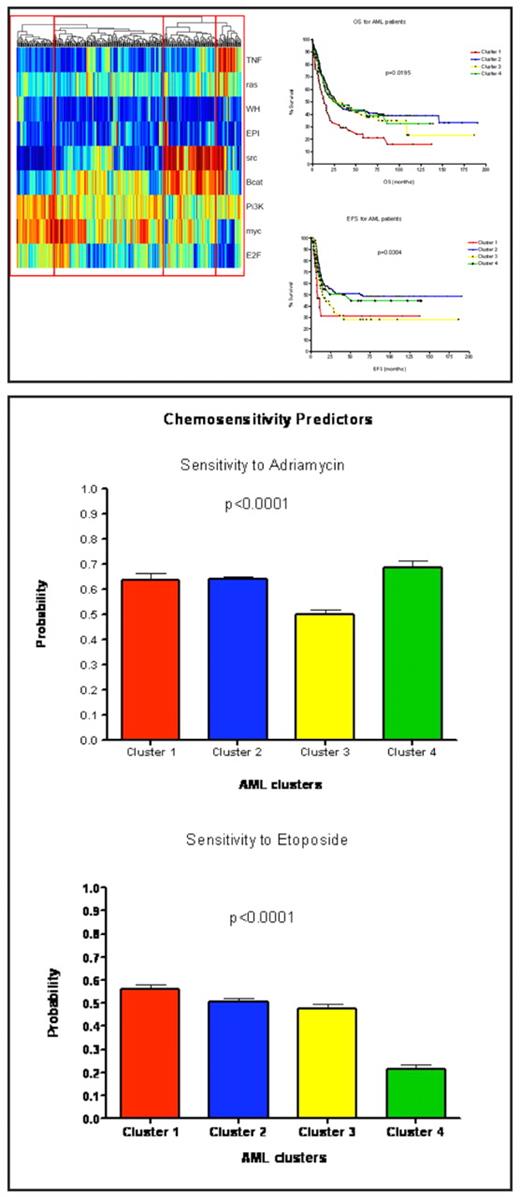
Gene expression profiling in acute myeloid leukemia (AML) patients has previously identified prognostically important clusters driven by the presence of chromosomal lesions, genetic mutations (CEBPA), or abnormal oncogene expression (EVI1). Several of these patients despite response to induction chemotherapy have poor disease-free survival (DFS), and relapse rates, thus emphasizing the need for novel therapeutic strategies. Gene expression analysis was conducted by applying signature profiles reflecting deregulation of oncogenic signaling pathways ( Myc, Ras, Src, PI3K), profiles representing an altered tumor environment (angiogenesis, TNF-α), and signatures of chemotherapy sensitivity (adriamycin and etoposide) to a cohort of 345 newly diagnosed AML patients previously treated on protocols of the Dutch–Belgian Hematology–Oncology Cooperative group and the Japan Adult Leukemia Study Group. Four distinct clusters were identified based on oncogenic signaling and tumor environment pathways. There was a statistically significant difference in overall survival (OS) (p < 0.0195) and DFS (p<0.0304) between the four groups. Patients in cluster 1 had the worst OS at 13.5 months and DFS of just 8.4 months. Patients in this cluster had increased activation of Myc and PI3K pathways and low levels of Src activation. Patients in clusters 2 and 4 had the best OS and DFS. Fifty-one percent of patients in cluster 4 had a nucleophosmin mutation (NPM1 +) and demonstrated higher activation of the Ras and TNF pathways compared to cluster 2, while patients in cluster 2 had increased activation of Myc when compared to Cluster 4. Interestingly, patients in Cluster 3 were most sensitive to the anthracycline, adriamycin, yet had a fairly poor DFS of just 14.4 months. While many biologic factors may play a role, one reason for poor DFS might be increased Src activation in this cluster and also much lower rates of NPM1 (12%) mutations. We could hypothesize that patients in this cluster might benefit from induction with chemotherapy in combination with a Src-inhibitor. This analysis has demonstrated distinct patterns of altered tumor biology and chemotherapy sensitivity amongst patients with clinically relevant subtypes of AML. Additional results evaluating chemosensitivity to cytarabine, and other nucleoside analogues are pending.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal