Abstract
BACKGROUND: Allogeneic hematopoietic stem cell transplantation (HSCT) for sickle cell disease (SCD) provides curative therapy but carries significant risk of transplant-related morbidity and mortality, graft failure, and disease recurrence. Past trials of myeloablative HSCT with busulfan in SCD patients showed a 10–13% rate of rejection and 8–9% rate of partial chimerism with busulfan steady-state concentration (Css) targeted to 400–600 ng/ml (area under curve (AUC) 575–877 mmol•min/L). In thalassemia and other diseases, total systemic exposure to busulfan is a critical determinant of engraftment yet is limited by risk of toxicity. Therapeutic drug monitoring (TDM) is used to measure variability in individual patient pharmacokinetics and allows dose adjustments to achieve desired level of busulfan exposure. A retrospective analysis of busulfan AUC in SCD patients during HSCT was performed to determine if higher systemic busulfan levels resulted in more effective engraftment or increased toxicities.
METHODS: A retrospective review of busulfan pharmacokinetics, engraftment status, and clinical toxicities in a cohort of SCD patients who received MSD bone marrow grafts at a single pediatric institution was performed. All patients received a myeloablative preparative regimen with busulfan (initially dosed for total 14 mg/kg), cyclophosphamide (200 mg/kg), antithymocyte globulin (90 mg/kg), and graft versus host disease prophylaxis with methotrexate and cyclosporine. Anticonvulsant prophylaxis with phenytoin or with levitiracetam plus lorazepam was given during busulfan administration. Busulfan was administered every 6 hours for 16 doses. Busulfan AUC was determined for the first dose and adjustments were made on subsequent doses if needed. Total AUC was calculated as a weighted average of total doses. Target AUC varied widely over the study period; however, for the last 3 years, the intended AUC has been targeted to 900–1100 mmol•min/L (Css 618–753 ng/ ml). Busulfan-associated toxicities recorded were: hepatic veno-occlusive disease (VOD), interstitial pneumonitis (IP), and seizure during or 1 day following busulfan administration.
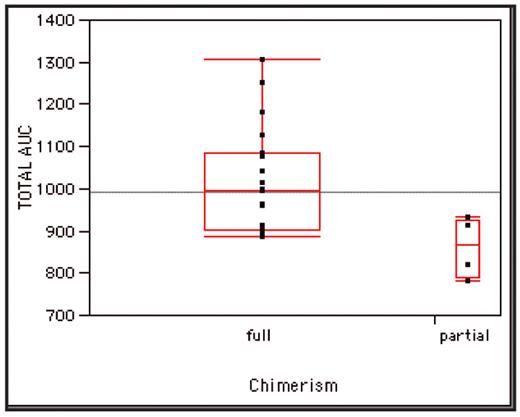
RESULTS: Twenty-seven consecutive hemoglobin SS SCD patients received HSCT with MSD between December 1993 and August 2007. All patients transplanted since May 1996 (n=25) had busulfan TDM performed. Median age was 8.8 years (range 3.3–17.4 years). Event-free survival was 96% with a median follow-up time of 3.9 years. Engraftment occurred in all cases with no subsequent graft rejection. Full donor chimerism (>95% donor leukocytes) was seen in 21 (84%) patients, and high partial donor chimerism (50–95% donor) in 4 (16%) at >12 months post-HSCT. Busulfan was administered orally in 18 (72%) and intravenously in 7 (28%). Dose adjustments were made in 17 patients (8 increased, 9 decreased). Median dose increase was 16.3% (range 9.7–76.5%), and median decrease was 14.3% (range 4.2–57.1%). Five (20%) patients had dose alterations >20% of initial dose (2 increased, 3 decreased). Median total dose received was 13.9 mg/kg (range 10.8–24.0). Median AUC for first dose was 968 mmol•min/L (range 595–1379) and median total AUC was 992 mmol•min/L (range 780–1305). Busulfan-associated toxicities were observed in 9 (36%) patients: 8 with mild-moderate VOD, 1 with IP. No seizures occurred during busulfan administration. Patients with busulfan-associated toxicity had mean total AUC of 1044.7 mmol•min/L (range 821–1305) versus 962.4 mmol•min/L (range 780–1184) in those without toxicity (p=0.185). Patients with partial donor chimerism had lower total AUC (mean 861.5 mmol•min/L, range 780–932) compared to those with full donor chimerism (mean 1017 mmol•min/L, range 885–1305; p=0.0379) (Figure). Since the implementation of a target busulfan AUC range of 900–1100 mmol•min/L, all patients (n=10) achieved full donor chimerism.
CONCLUSIONS: We conclude that higher busulfan levels are associated with decreased incidence of partial donor chimerism in SCD patients during myeloablative HSCT with MSD bone marrow grafts. A target AUC range of 900–1100 mmol•min/L, attainable with TDM and dose adjustment, provides effective engraftment without increased risk of busulfan-related toxicity.
Total busulfan AUC in patients with full donor chimerism (mean 1017 mmol•min/L) vs. partial chimerism (mean 861.5 mmol•min/L, p=0.0379).
Total busulfan AUC in patients with full donor chimerism (mean 1017 mmol•min/L) vs. partial chimerism (mean 861.5 mmol•min/L, p=0.0379).
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal