Abstract
We previously reported 2-year overall survival (OS) of 65% among 33 pts with MCL given nonmyeloablative HCT (
long-term disease control and
resolution of chronic GVHD.
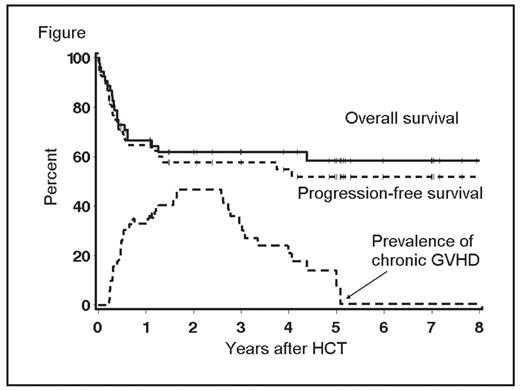
Pts were conditioned with 2Gy TBI with or without fludarabine (90 mg/m2). Median age for all pts was 56 (range 33–75) years and median number of prior regimens was 4. Forty percent of pts had failed high-dose autologous HCT and an additional 11% had planned autologous HCT before allograft (4 pts had refractory disease and 2 were in PR). Comorbidity scores of ≥3 were found among 40% of pts. Forty percent of pts were not in CR/PR at HCT and 26% and 21% had marrow infiltration and lymph node size ≥5 cm, respectively. Donors were related (n=28) or unrelated (n=25). After HCT, incidences of grades II, III, and IV acute GVHD were 25%, 13%, and 9% respectively, and chronic extensive GVHD was 53%. Complete (CR) and partial remissions (PR) were seen in 71% and 3% of pts with measurable disease at HCT, respectively. Estimated 5-year rates of non-relapse mortality (NRM), progression/relapse, OS, and progression-free survival (PFS) were 27%, 22%, 58%, and 52%, respectively (Table 1). Among 19 pts in CR at HCT, 11 are alive and in CR, 7 died in CR, and one relapsed (now in CR after Rituximab and donor lymphocyte infusion). Among 13 in PR at HCT, 10 achieved CR and are alive, one died in PR, and 2 died from relapse. Among 21 pts with refractory/relapsed disease at HCT, 12 achieved CR and are alive, 2 have stable disease and are alive, and 7 relapsed (2 are alive in CR and PR after further treatment). At 5-years, 44% and 14% were alive without or with chronic GVHD requiring immunosuppression (Figure); and median duration of treatment for chronic GVHD was 33 months. Outcomes were comparable among related and unrelated recipients. Relapse rates were 47% vs. 14% among pts with vs. without LN size of ≥5 cm (p=0.02) and NRM was 41% vs.17% (p=0.05) among pts with HCT-CI scores of ≥3 vs. 0–2, respectively. Nonmyeloablative HCT is a potentially curative therapeutic modality for pts with advanced MCL, including patients who were chemotherapy-refractory, with a median PFS beyond 5 years. Sustained remissions and continuing resolution of chronic GVHD were observed with extended follow up. Pts with bulky LN might benefit from further debulking strategies before HCT.
Table: Outcomes by donor type
| . | Donor . | |
|---|---|---|
| . | Related (n = 28) . | Unrelated (n = 25) . |
| . | % . | % . |
| Grades III–IV non-hematological toxicities | 39/18 | 38/26 |
| Acute GVHD, grades II/ III/ IV | 22/14/7 | 28/12/12 |
| Chronic GVHD | 50 | 55 |
| CR | 73 | 67 |
| 5-year NRM | 26 | 28 |
| 5-year Progression/relapse | 22 | 21 |
| 5-year PFS | 53 | 51 |
| 5-year OS | 59 | 56 |
| 5-year Pts alive with chronic GVHD requiring immunosuppression | 14 | 7 |
| . | Donor . | |
|---|---|---|
| . | Related (n = 28) . | Unrelated (n = 25) . |
| . | % . | % . |
| Grades III–IV non-hematological toxicities | 39/18 | 38/26 |
| Acute GVHD, grades II/ III/ IV | 22/14/7 | 28/12/12 |
| Chronic GVHD | 50 | 55 |
| CR | 73 | 67 |
| 5-year NRM | 26 | 28 |
| 5-year Progression/relapse | 22 | 21 |
| 5-year PFS | 53 | 51 |
| 5-year OS | 59 | 56 |
| 5-year Pts alive with chronic GVHD requiring immunosuppression | 14 | 7 |
Figure
Disclosures: Off Label Use: Fludarabine and 2Gy Total body irradiation were used for conditioning before HCT.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal