Abstract
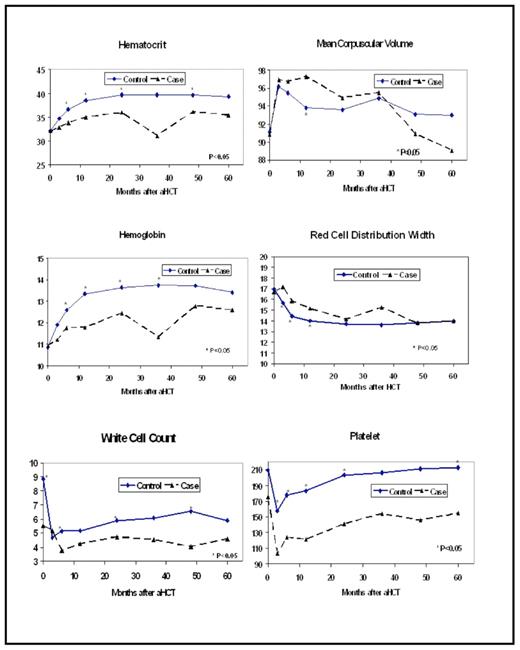
t-MDS/AML is the most common cause of non-relapse mortality in patients undergoing autologous hematopoietic cell transplantation (aHCT) for Hodgkin lymphoma (HL) or non-Hodgkin lymphoma (NHL). Although t-MDS/AML is known to result from damage to hematopoietic stem cells (HSC) as a result of genotoxic cancer treatment, the sequential cellular and molecular changes leading to its development are not clearly defined. To better understand the pathogenesis of t-MDS/AML, we conducted a prospective study in 179 patients undergoing aHCT for HL (n=41) or NHL (n=138) between 1999 and 2004, who participated in a prospective longitudinal study from pre-aHCT to five years post-aHCT, with a serial collection of bone marrow and peripheral blood samples. The median length of follow-up for this cohort was 3.9 years. This report focuses on alterations in peripheral blood parameters from pre-aHCT to the development of t-MDS/AML, and compares these trends with the patients in this cohort who did not develop t-MDS/AML. A total of 22 patients have developed t-MDS/AML in this longitudinally followed cohort thus far, resulting in a cumulative incidence of 11% at 5 years. Serial evaluation of peripheral blood parameters including hematocrit, mean corpuscular volume (MCV), hemoglobin (HGB), red cell distribution width (RDW), white blood cell (WBC) count, and platelet (PLT) count, were abstracted from medical records for the following time points: pre-aHCT, day 100, 6 month, 1 year, 2 year, 3 year, 4 year and 5 year after aHCT, for a total of 1129 time points. Values of peripheral blood parameters associated with post-aHCT relapse or persistence of the primary lymphoma or from 3 months prior to development of t-MDS/AML, were excluded from analysis. As shown in the Figure, comparison of the peripheral blood parameters in subjects who developed t-MDS/AML (cases; n=22) with those who did not (controls; n=157) revealed that hematocrit values were lower for cases compared to controls at all post-aHCT time points. HGB values were lower among cases compared to controls at all post-aHCT time points. The RDW values were higher for cases compared to controls at day 100, 6 months and 1 year post-aHCT. MCV values did not differ between cases and controls at any of the time points. WBC counts for the cases were lower than controls pre-aHCT and also at all time points from 6 months post-aHCT onwards. PLT counts for cases were lower than controls at all time points pre- and post-aHCT. A fixed effect growth curve model was fitted to the data from day 100 to 5 years post-aHCT after adjusting for age at aHCT, primary diagnosis, race/ethnicity, and sex, to examine the rate of change in the peripheral blood parameters over time. Results revealed a significantly sharper decline in MCV for cases (β per 100 days = −0.43) over time as compared to controls (β =−0.15; p = 0.006). Although hematocrit increased with time for both cases and controls, the slope for the cases was significantly less steep (controls: β per 100 days=0.31 vs. cases: β per 100 days=0.12; p =0.01). In summary, we consistently observed lower values for red cell parameters, WBC, and platelets in patients with t-MDS/ AML as compared to controls across multiple timepoints post-aHCT. These differences appeared soon after HCT, were persistent, and preceded the development of t-MDS/AML. Our previous studies indicate that there is increased turnover and reduced regenerative capacity of premalignant hematopoietic stem cells at early stages of development of t-MDS/AML. The early and persistent reduction in peripheral blood parameters observed here provides further evidence that bone marrow injury and ineffective hematopoiesis long predate the development of t-MDS/AML after aHCT. Poor hematocrit recovery and enhanced decline in MCV after aHCT were independently associated with increased risk of t-MDS/AML and warrant further development as readily applied biomarkers for disease and the need for close monitoring.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal