Abstract
B-chronic lymphocytic leukemia (B-CLL) cells express tumor associated antigens that may generate a T cell mediated immune response, but present these antigens poorly. Moreover, patients with B-CLL often have poor immune function due to the disease or its treatment. We have shown that expression of transgenic CD40L increases the immunogenecity of human B-CLL cells ex vivo and in vivo, and that this effect can be potentiated by co-expression of transgenic IL2. Previous studies described outcomes when adenoviral vectors were used to obtain gene transfer, but because of the complexities and expense of manufacture of viral vectors, and their lingering safety concerns, we determined whether it was possible to use electroporation (with the MaxCyte device) as a physical means of transferring CD40L and IL2 plasmids to produce vaccines with similar biological properties in vitro and in vivo. Table 1 compares the phenotype of the vaccines using each vector.
Table 1. Comparision of immunogenic characteristics and viability of the adenoviral and plasmid vaccines
| Type of Vaccine . | CD40L (%) . | CD80 (%) . | CD86 (%) . | IL-2 (pg/ml/10e6 cells) . | Viability (%) IL2 CD40L . | |
|---|---|---|---|---|---|---|
| All the values are given as mean ± SE. * P< 0.01, Paired Student’s t test. | ||||||
| Adenoviral | ||||||
| Pre | 0.2 ± 0.01 | 2.6 ± 2.4 | 7.5 ± 3.9 | |||
| Post | 66.1 ± 5.5* | 50.2 ± 7.8* | 69.5 ±11* | 253.5 ± 82.6 | 93.6 | 94.2 |
| Plasmid | ||||||
| Pre | 1.3 ± 0.85 | 11.5 ± 6.2 | 19.7 ± 6.8 | |||
| Post | 55.5 ± 5.1* | 19.2 ± 9.3 | 26.4 ± 9.7 | 4806.6 ±1398.9 | 84.4 | 88.4 |
| Type of Vaccine . | CD40L (%) . | CD80 (%) . | CD86 (%) . | IL-2 (pg/ml/10e6 cells) . | Viability (%) IL2 CD40L . | |
|---|---|---|---|---|---|---|
| All the values are given as mean ± SE. * P< 0.01, Paired Student’s t test. | ||||||
| Adenoviral | ||||||
| Pre | 0.2 ± 0.01 | 2.6 ± 2.4 | 7.5 ± 3.9 | |||
| Post | 66.1 ± 5.5* | 50.2 ± 7.8* | 69.5 ±11* | 253.5 ± 82.6 | 93.6 | 94.2 |
| Plasmid | ||||||
| Pre | 1.3 ± 0.85 | 11.5 ± 6.2 | 19.7 ± 6.8 | |||
| Post | 55.5 ± 5.1* | 19.2 ± 9.3 | 26.4 ± 9.7 | 4806.6 ±1398.9 | 84.4 | 88.4 |
Vaccines made by both approaches met the release criteria for CD40L and IL2 expression (CD40L ≥20% and IL-2 ≥ 150 pg/ml/1x10e6 cells ), but expression of IL2 was higher in the plasmid vaccines, expression of CD40L was equivalent in each and expression of the additional co-stimulatory molecules CD80 and CD86 (induced after CD40 activation by transgenic CD40L) was higher in the adenoviral vaccines. Fourteen patients were given adenoviral-vaccines and nine the plasmid transduced cells. Each of these patients received up to 18 s.c. injections of IL-2 secreting and CD40L expressing tumor cells. Both types of vaccine were well tolerated. Table 2 shows the results of culturing patient T cells with autologous B-CLL tumor cells.
Table 2. Comparision of anti-B-CLL T cell responses induced by adenoviral and plasmid vaccines
| Type of Vaccine . | Pre-vaccine . | After 3rd vaccine . | After 6th vaccine . |
|---|---|---|---|
| All the values were are given as mean ± SE. *P<0.05, Wilcoxon Signed Ranks test | |||
| Adenoviral | 307.3 ± 293.9 | 375 ± 306.8 | 656.8 ± 373.8 |
| IFN-γ spots/10e6 T cells IL-5 spots/10e6 T cells | 0 | 12.8 ± 7.9 | 5.8 ± 2.3 |
| Plasmid | 31.1 ± 14.8 | 38 ± 17.8 | 32.9 ± 19.5 |
| IFN-γ spots/10e6 T cells IL-5 spots/10e6 T cells | 4 ± 2.7 | 14 ± 10.2 | 203.9 ± 156.3* |
| Type of Vaccine . | Pre-vaccine . | After 3rd vaccine . | After 6th vaccine . |
|---|---|---|---|
| All the values were are given as mean ± SE. *P<0.05, Wilcoxon Signed Ranks test | |||
| Adenoviral | 307.3 ± 293.9 | 375 ± 306.8 | 656.8 ± 373.8 |
| IFN-γ spots/10e6 T cells IL-5 spots/10e6 T cells | 0 | 12.8 ± 7.9 | 5.8 ± 2.3 |
| Plasmid | 31.1 ± 14.8 | 38 ± 17.8 | 32.9 ± 19.5 |
| IFN-γ spots/10e6 T cells IL-5 spots/10e6 T cells | 4 ± 2.7 | 14 ± 10.2 | 203.9 ± 156.3* |
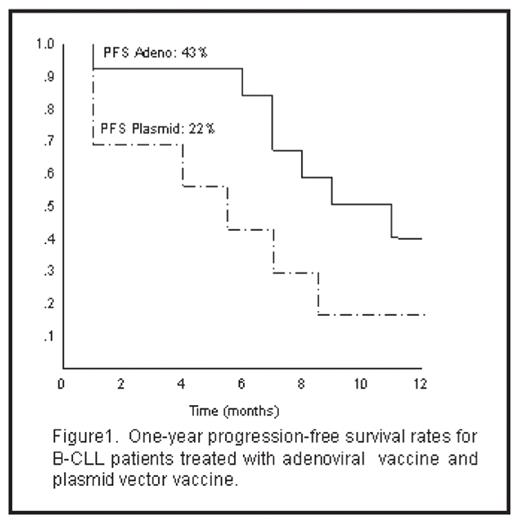
After 3 and 6 injections, both the adenoviral and plasmid vaccines had induced a rise in spot forming cells (SFC) for IL5, a cytokine associated with Th2 cells, but the rise was greatest in the recipients of the electroporated plasmid vaccine. By contrast, only the adenoviral vaccine induced a rise in SFC that produced IFN-γ, a cytokine associated with Th1 cells. Studies using MHC class I and II blocking antibodies showed that the IL5 and IFN-γ responses to both types of vaccine were mediated by HLA restricted T lymphocytes. The 1-year progression-free survival rates (PFS) for adenoviral vaccine group and plasmid vector group were 43% and 22% respectively. Figure 1 shows 1-year PFS rates for each group. Hence electroporation provides a more rapid and simpler means of preparing IL2/CD40L expressing B-CLL vaccines, but the cells express higher levels of IL2 and lower levels of “secondary” co-stimulator molecules than adenoviral vaccines, and produce an anti-tumor immune response of different polarity. Currently, we are evaluating electroporation of mRNA encoded CD40L which appears to augment upregulation of additional costimulatory molecules.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal