Abstract
Background: Gene expression profiling molecular classification of MM was proven to be an independent predictor of survival post autologous stem cell transplant (ASCT); however it had limited clinical applicability due to its complex methodology and high costs. We have previously reported the results of a protein-array based classification of MM in an initial testing cohort and concluded that positive immunoperoxidase staining for FGFR3, Cyclin B2 or Integrin beta7 correlates with a shortened survival post ASCT (
Methods: Immunoperoxidase staining for Cyclins B1, B2, D1, D2 and D3, FGFR3, PAX5 and Integrin beta 7 were previously validated in our initial testing cohort (n=52). Further analysis of our initial testing cohort identified 3 risk groups: positive expression of FGFR3 or Integrin beta 7 defined as “High risk”, positive Cyclin B2 (in the absence of FGFR3 or Integrin beta 7) as “Intermediate risk” and the lack of expression of any of these biomarkers defined as “Low risk”. In order to confirm the predictive value of our proposed protein-array classification, these immunohistochemical (IHC) stains were performed on the bone marrow biopsies of a larger and independent validation cohort of 79 newly diagnosed MM patients uniformly treated with a dexamethasone based regimen followed by ASCT. The clinical parameters, response criteria and survival outcomes (TTP and OS) of this validation cohort were defined according to the international uniform response criteria. For IHC analysis two pathologists who were blinded with regards to the clinical outcome of these patients scored the cases independently as positive or negative. Discordance in their scoring was seen in 20/79 (25.7%) with a consensus scoring reassigned to all of these cases. The Kaplan-Meier method was used to estimate OS and TTP. Multivariate analysis was performed using the Cox regression method.
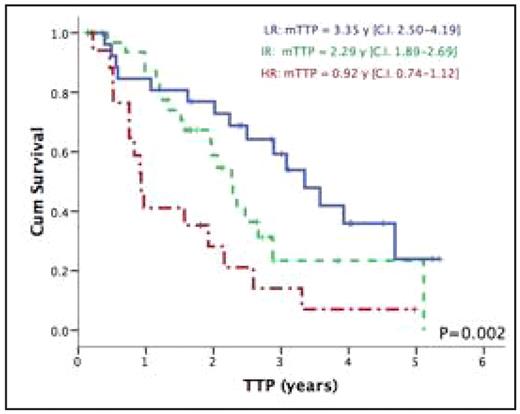
Results: 79 patients were included in this validation cohort, the median age was 54.4 yrs (27.9–71), 23.7% had ISS stage III, median beta 2-microglobulin was 3.29 mg/L (1.16–37.5). Del13q and t(4;14) were detected by FISH in 35.6% and 13.6% of patients, respectively. Post ASCT, 68% achieved a CR or VGPR with an overall median TTP and OS of 2.29 years (CI 1.84–2.73) and 5.74 years (CI 4.98–6.51) respectively. Expression of FGFR3 was detected in 7.6% of the patients, cyclin B2 in 58.2% and integrin-beta7 in 17.7%. In univariate analysis expression of FGFR3 was associated with a significantly shorter TTP (P=0.011) but not OS (P=0.114). Similarly integrin-beta7 predicted for a shorter TTP (P=0.008) but not OS (P=0.570). Cyclin B2 also predicted for worse TTP (P=0.047) but not OS (P=0.098), whereas the expression of cyclins D1, D2, D3 and PAX5 did not affect survival. Based on our testing cohort definition of risk groups, 18/79 (22.8%) were considered as “High risk” with significantly shorter TTP 0.93 years (CI 0.74–1.12) compared to 2.29 years (CI 1.88–2.69) and 3.35 years (CI 2.51–4.19) for the “Intermediate” (34/79; 43%) and “Low” (27/79; 34.2%) risk groups respectively (P=0.002). The 5-years estimates for OS was 57.1% for the High-risk group compared to 66.3% and 71.6% for the Intermediate and Low risk group respectively (P=0.258). Multivariate analysis was performed using ISS, del13q and the TMA risk group classification as variables. The TMA classification and del 13q were the only independent predictors of TTP with the high-risk group having 3.4 fold greater risk of relapse (P=0.001).
Conclusion: We have validated our protein array based classification of Multiple Myeloma and confirmed its survival predictive value post ASCT. MM patients with the High-risk signature should be spared the toxicity of ASCT and considered instead for other frontline novel therapeutic agents.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal