Abstract
Implementation of transplant procedures and the availability of alternative sources of stem cells for patients without HLA identical siblings has made allogeneic stem cell transplant (ASCT) a suitable therapy for many patient affected by acute myeloid leukemia (AML); consequently, in AML patients transplant strategies must be carefully designed according to stringent risk/benefit analysis. To date, risk assessment relies on identification of baseline prognostic parameters such as karyotype, which has become critical in order to choose post-remissional therapy. Furthermore, there are strong evidences that the presence of specific gene abnormalities, such as mutations of FLT3, NPM and c-kit, allow to identify, within homogeneous cytogenetic groups, subsets of patients with distinct clinical outcome. We hypothesized that, in order to guide the post-remission decisional process, more robust informations may derive from the combined evaluation of conventional baseline and delayed prognosticators. Minimal residual disease (MRD) detection is potentially the most efficient tool to investigate the quality of remission and might represent the ideal parameter to be associated with baseline biological features for prognostic purposes. Therefore, we aimed to determine whether combined analysis of karyotype and MRD, using multiparametric flow-cytometry (MPFC), could help to refine risk assessment in adult patients with AML, allowing the most appropriate post-remissional strategy to be selected. We analyzed 132 patients with AML who had intensive chemotherapy with EORTC/GIMEMA protocols. According to MRC classification, 22 (17%), 104 (78%) and 6 (5%), respectively, had good, intermediate and poor risk karyotype. Thirteen of 107 (12%) carried a FLT3-ITD. Within good and intermediate risk categories, MRD positivity (≥ 3.5×10−4 residual leukemic cells at the post-consolidation time-point) was associated with a worse prognosis in terms relapse free (RFS) and overall survival (OS). In fact, we observed that:
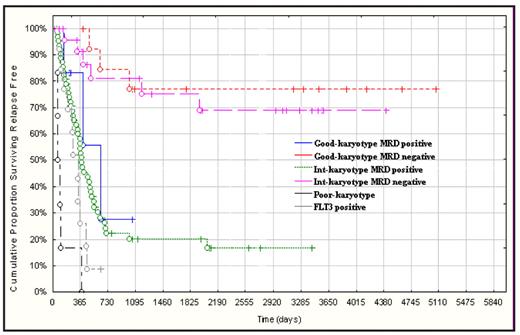
MRD negative good and intermediate risk categories shared the same favorable prognoses with RFS and OS of 80% vs. 69% and 84% vs 54%, respectively;
MRD positive good and intermediate risk categories fared as worse as those with poorrisk karyotype or FLT3-ITD in terms of RFS (28% vs. 17% vs. 0%) and OS (42% vs. 21% vs. 17%).
Using this approach the conventional cytogenetic classification that uses three categories is simplified into 2 prognostically defined groups:
favorable, including MRD negative good and intermediate risk karyotype;
unfavorable, including MRD positive good and intermediate risk karyotype, poor risk karyotype and FLT3-ITD positive cases (Fig. 1).
Based on these observations, we believe that ASCT is recommended, not only for poor-risk karyotype or FLT3 positive AML, but also for good/intermediate risk categories not gaining MRD negativity, being this option able to provide a superior chance of prolonged RFS (Maurillo et al, JCO 2008). On the other hand, patients belonging to MRD negative good/intermediate risk categories, who can experience a 5-years survival higher than 60%, may have their life expectancy hampered by the choice of a therapeutic strategy with a disadvantageous risk/benefit ratio: for this category a standard intensification procedure (chemo and/or autologous transplant) is indicated. In conclusion, the combined assessment of baseline prognosticators (cytogenetics) and parameters inherent the quality of response (MRD), is useful to define discrete categories of risk within the respective karyotypic groups, allowing tailored therapeutic approaches to be applied according to the actual clinical risk and avoiding under or over treatments.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal