Abstract
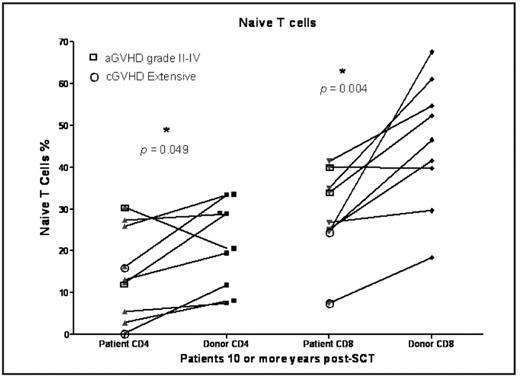
After allogeneic stem cell transplantation (SCT), there is a prolonged immune deficiency and delayed T cell reconstitutions results in significant morbidity and mortality. However limited data are available on immune reconstitution in patients surviving beyond a decade following SCT. Four hundred sixty two patients with hematological malignancies received SCT from an HLA identical sibling in our institute between 1993–2004. Of these, 110 patients 3 or more years post-transplantation, prospectively enrolled in a long-term evaluation protocol. Twenty one of these survived more than 10 years post SCT (median follow-up 11.8 y range 10–14.75y). Diagnoses included chronic myelogenous leukemia (17), acute myelogenous leukemia and myelodysplastic syndrome (3), and multiple myeloma (1). We studied T cell reconstitution in these patients and compared it to samples from their stem cell donors cryopreserved at time of transplant. There was no difference of age at SCT in patients (median age 35.5, range 13–56y) and in the donors (median age 34, range 14–58y). All patients received cyclophosphamide and 13.6 Gy total body irradiation. Patients received T cell depleted bone marrow (n=15) or peripheral blood SCT (n=6) with cyclosporine GVHD prophylaxis and delayed add-back of donor lymphocytes 30–90 days post transplant. Six (29%) developed acute GVHD (grade II–IV) and 18 (86%) chronic GVHD (13 limited, 5 extensive). Six (29%) patients received immunosuppressive therapy (IST) for cGVHD beyond 3 years from SCT but all were off immunosuppressive treatment at the time of study. In the 21 patients there were no significant difference in the absolute lymphocyte, neutrophil or monocyte count, compared with the donor pre-transplant absolute counts of circulating NK and T cell subsets, and B cells were measured using multicolor flow cytometric analysis in 9 patient-donor pairs. Patients had fewer naïve CD4 (p = 0.049) and naïve CD8 (p = 0.004) T cells, fewer CD4 central memory T cells (p = 0.03), fewer CD56 [int] CD16-NKG2A+2D+ NK cells (p = 0.02); and more effector CD8+ T cells (p = 0.04) in patients compared to their donors. ALC and FoxP3+ regulatory T cells were not significantly different between the patients and their donors. The T cell receptor excision circles (TRECs) and T cell receptor repertoire analyses to evaluate thymic function and T cell regeneration is ongoing. In conclusion, patients surviving 10 or more years after allogeneic SCT still show a deficit in the naïve and central memory post-thymic compartment. However these abnormalities appear to be compatible with good health.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal